Lysine-polymer Conjugation Technologies
Polypeptides have been frequently used as building blocks for the preparation of amphiphilic block copolymers. In contrast to proteins, peptides provide shorter chain lengths, lower molecular weights and less complex tertiary structures. In the past two decades, self-assembly of polypeptide-based block copolymers into micelles and vesicles has been intensively explored, particularly for applications in catalysis and drug delivery. BOC Sciences is committed to providing flexible biomolecule-polymer conjugates for global customers to achieve new drug discovery milestones with comprehensive and advanced platforms. Our polymer bioconjugation service platform includes nucleic acid-polymer conjugation, protein-polymer conjugation, enzymes-polymer conjugation, liposome-polymer conjugation and carbohydrate-polymer conjugation.
Introduction of Lysine-polymer Conjugates
The first-generation methods that were developed to create protein/peptide polymer hybrids under mild chemical conditions were based on reactions between the activated hydroxyl group of poly(ethylene glycol) (PEG) chains and primary amines from proteins. The latter groups are abundantly present on the surface of proteins in both lysine residues and the N-terminus. This facilitates easy functionalization by both alkylation, which maintains the positive charge of the protein at physio-logical pH, and acylation, which is accompanied by loss of charge at the conjugation site. Generally speaking, these first-generation methods are non-specific and result in the attachment of polymers at multiple sites on the protein. Nevertheless, this type of modification, also known as PEGylation, has been widely employed since the 70s to reduce the limitations of protein/peptide-based drugs, such as a short half-life and immunogenicity. PEGylation leads to an increased water solubility and stability, prevents clearance through the kidneys, and hence gives rise to a prolonged plasma lifetime.
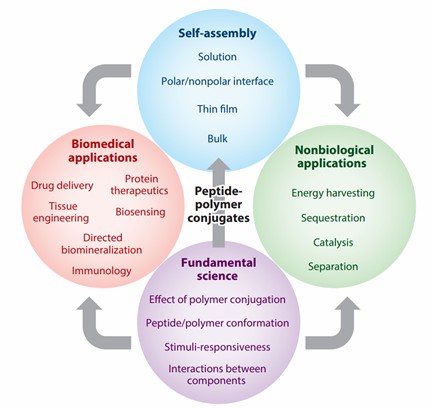 Fig. 1. Applications of peptide-polymer conjugates (Annu. Rev. Phys. Chem. 2013, 64: 631-57).
Fig. 1. Applications of peptide-polymer conjugates (Annu. Rev. Phys. Chem. 2013, 64: 631-57).
Our Lysine-polymer Conjugation Strategies
Active Site Blocking Strategies
One possibility to get better selectivity over the synthesis of the bioconjugates is to shield the active site of the enzyme or the recognition area of the protein during the coupling reaction. To achieve this, an inhibitor, a substrate or a ligand can be used to cover the reactive groups in the sensitive areas. For example, avidin was PEGylated in the presence of a biotin-PEG conjugate as a protective agent. Shielding of avidin's biotin binding site proved effective during conjugation of large PEG chains (10 and 20 kDa). However, in the case of smaller PEG chains (5 kDa) this protection strategy had no positive effect on the affinity of the resulting avidin conjugate. As a large chemical service organization, BOC Sciences is dedicated to providing polymer modification and synthesis services for drug development and drug delivery programs. We are capable of completing small (mg) and large (g) quantities of polymer products and transferring these technologies to a bulk manufacturing plant. To ensure quality, all final products will be thoroughly analyzed by our advanced technologies, including mass spectrometry and chromatography.
Solid Phase Peptide Synthesis Strategies
In solid phase peptide synthesis (SPPS), an amino acid is first anchored with its C-terminus to a swollen crosslinked polystyrene resin. In suspension, sequential deprotection and addition of N-protected amino acids affords a growing peptide chain in a stepwise fashion. Cleavage from the resin support finally affords the final product. Because the solid support can be purified from unreacted substrates and by-products by simple filtration, coupling reactions can be driven to high conversions by using large excesses of reagents. Furthermore, as all reactive amino acid side groups are protected during the synthesis, selectivity problems are avoided. In this way, the N-terminus of the synthetic peptide can not only be reacted with an amino acid, but can also be specifically coupled with a polymer chain functionalized with a carboxylic acid or succinimidyl moiety. Scientists from BOC Sciences use solid phase peptide synthesis technology to provide global customers with cost-effective protein-polymer conjugates and peptide-polymer conjugates to promote customers' R&D progress in new drug development, material production and other industries.
Reductive Alkylation Strategies
As the ε-amine group on lysine and the primary amine at the N-terminus have different pKa (about 10.0-10.2 and 7.6-8.0, respectively), their difference in reactivity under slightly acidic conditions can, in principle, be utilized for the selective functionalization of the N-terminus with the activated esters. Reductive alkylation proves to be guite efficient. When comparing immobilization methods for human serum albumin (HSA) to monoliths composed from methacrylate-based polymers, the reductive alkylation protocol resulted in a more efficient immobilization than the epoxy, succinimidyl carbonate and carbodiimide methods. These columns were subsequently used to separate both racemic warfarin and tryptophan, where the column prepared by reductive alkylation was found to give the best resolution. BOC Sciences has built a world-leading polymerization technology platform. In addition to providing specialized polymer synthesis and polymer modification services, we also offer polymer isolation and purification services, including precipitation classification, extraction classification, extraction method, ion exchange resin method, reprecipitation method, etc.
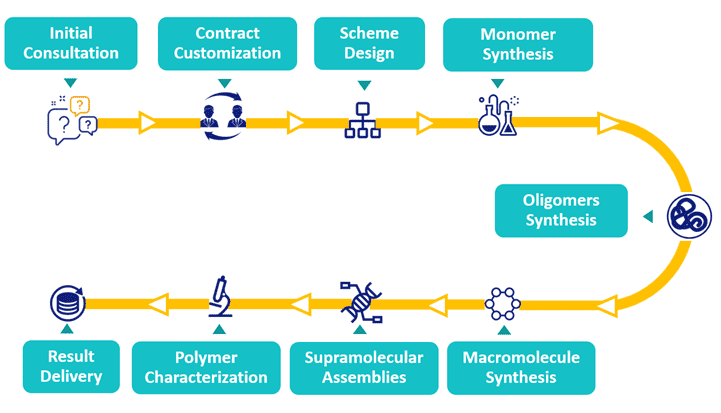
Our Polymer Bioconjugation Workflow
References
- Klok, H.A. et al. Peptide/protein-polymer conjugates: synthetic strategies and design concepts. Chem. Commun. 2008, 2591-2611.
- Hest, J. et al. Polypeptide-polymer bioconjugates. Chem. Soc. Rev. 2010, 39: 329-353.

 Fig. 1. Applications of peptide-polymer conjugates (Annu. Rev. Phys. Chem. 2013, 64: 631-57).
Fig. 1. Applications of peptide-polymer conjugates (Annu. Rev. Phys. Chem. 2013, 64: 631-57). 

