Browse by
Introduction

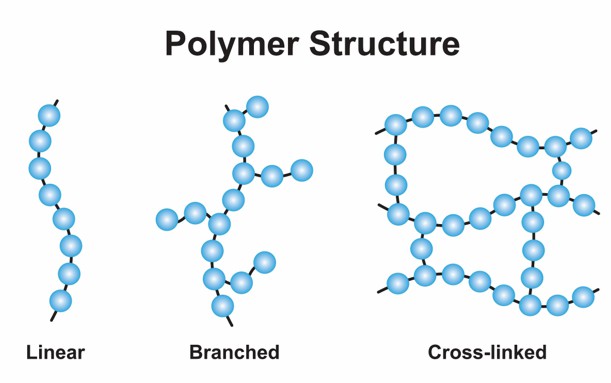
Polymers are extremely large molecular weight macromolecules formed by small molecular repeating units bonded by covalent bonds, and were introduced by Staudinger after 1920. The molecular weight of the polymers can reach hundreds of thousands. Polymer-based materials are playing an increasingly important role in today's society and are an integral part of our lives. According to the main chain structure, polymers can be divided into three main groups: carbon chain polymers, hetero-chain polymers and elemental polymers. Carbon chain polymers refer to polymers whose main chain consists entirely of carbon atoms, such as polyethylene, polypropylene, etc. In addition to carbon atoms, the main chain of hetero-chain polymers also contains oxygen, sulfur and other heteroatoms, such as polyethers. Elemental organic polymers have no carbon atoms in the macromolecular backbone and are mainly composed of atoms such as silicon and boron, such as polysiloxanes.
Polymer Synthesis Strategies
According to the polymerization mechanism, the reactions for synthesizing polymers are divided into two categories, step-growth polymerization and chain polymerization. Most polycondensation and polyaddition reactions are step growth polymerization, characterized by the slow and gradual transformation of low molecules into high molecules. In contrast, the reaction of alkene monomers such as ethylene to form polymers is a chain polymerization.
Applications of Polymers
The mechanical properties of resins are distributed between rubber and fibers, from soft plastics close to rubber to hard plastics close to fibers. For example, polyethylene is generally a soft plastic, while polystyrene is a hard plastic. In polymer synthesis products, plastic output accounts for nearly 2/3, including general-purpose plastics such as polyethylene and polypropylene, and engineering plastics such as polycarbonate.
Synthetic rubber has high elasticity, and a small force can produce a large deformation (500% to 1000%). Synthetic rubbers are generally nonpolar, amorphous, high molecular weight polymers. It has flexible molecular chains that are coiled at room temperature. When stretched, the molecular chain elongates and the degree of order increases. Synthetic rubber generally includes styrene-butadiene rubber (SBR), nitrile butadiene rubber (NBR), etc.
Compared with rubber, fiber has the characteristics of small deformation, small elongation, high modulus and tensile strength. The polymers that make up the fibers generally have polar groups and high crystallinity. The melting point of the fiber is generally above 200 °C, which is conducive to hot water washing and ironing. Currently, polyester, nylon and acrylic are the three major categories of fiber varieties in the world.
If you are interested in our polymer products, please contact us immediately!


 Acid Functional Polymers & Salts
Acid Functional Polymers & Salts
 Acrylic Polymers
Acrylic Polymers
 Alkene & Vinyl Polymers
Alkene & Vinyl Polymers
 Amide and Imide Polymers
Amide and Imide Polymers
 Amine Functional Polymers & Salts
Amine Functional Polymers & Salts
 Biodegradable Polymers
Biodegradable Polymers
 Copolymers
Copolymers
 Dendrimers
Dendrimers
 Epoxy Polymers
Epoxy Polymers
 Halogenated Polymers
Halogenated Polymers
 Natural Polymers & Derivatives
Natural Polymers & Derivatives
 Poly(ethylene glycol)s (PEGs) & Derivatives
Poly(ethylene glycol)s (PEGs) & Derivatives
 Polyester
Polyester
 Polyether
Polyether












