- CAS: 25038-54-4
- Molecular Weight: 339.47
- Molecular Formula: C18H33N3O3X2
- CAS: 25085-02-3
- Molecular Weight: 166.13
- Molecular Formula: C6H9NNaO3
- CAS: 25086-89-9
- Molecular Weight: ~25000
- Molecular Formula: (C6H9NO.C4H6O2)x
- CAS: 25189-55-3
- Molecular Formula: (C6H11NO)n
- CAS: 25805-17-8
- Molecular Formula: [N(COC2H5)CH2CH2]n
- CAS: 28107-09-7
- Molecular Formula: C7H7NO
- CAS: 28408-65-3
- Molecular Weight: 85.1045
- Molecular Formula: C4H7NO
- CAS: 645-93-2
- Molecular Weight: 128.0896
- Molecular Formula: C3H4N4O2
- CAS: 9003-05-8
- Molecular Formula: [CH2CH(CONH2)]n
Introduction

Amide polymers are macromolecular polymers containing amide bonds (-CONH-), which are very important basic unit in polymer synthesis chemistry and biology. There are many synthetic methods for amide bonds, but the condensation reaction of acids and primary amines is one of the most commonly used methods for the preparation of amide bonds. In addition, amide bonds can also be obtained by reacting esters, aldehydes and alcohols with amines or ammonium salts.[1] Due to the particularity of the chemical structure, hydrogen bonds can be formed between amide bonds. The existence of hydrogen bonds has a great influence on the aggregation state and performances of polymer materials. Therefore, amide bonds with inherent hydrogen bonds have attracted much attention. Many polymers are modified or functionalized by introducing amide bonds.
In the field of synthetic chemistry, amide polymers mainly include polyamide (PA) and polyester amide. Polyamide, also known as nylon, is a high molecular polymer composed entirely of amide bond. The existence of a large number of amide bonds on the main chain of polyamide produces dense hydrogen bonds, which greatly increases the intermolecular force, and also makes the molecular chain arrangement more compact and orderly, thereby forming a crystal structure.
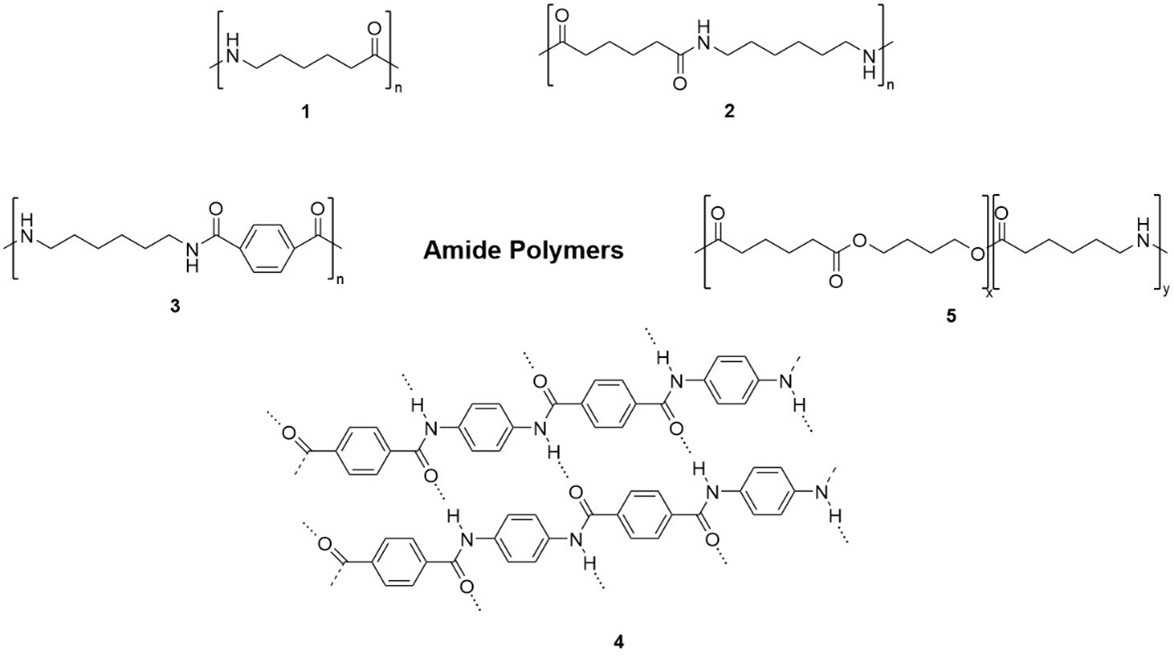 Fig. 1. Examples of amide polymers.
Fig. 1. Examples of amide polymers.
Application
Common polyamides can be divided into:
- Aliphatic Polyamides. For example, PA6 (Fig 1. Compound 1) are polymerized from caprolactam monomer; PA66 (Fig 1. Compound 2) is polymerized from adipic acid monomer and hexamethylene diamine monomer, which is widely used in engineering plastics, fiber and other fields.
- Semi Aromatic Polyamides. For example, PA6T/66 (Fig 1. Compound 3) is p- phenylene adipamide polymer. The synthesized PA6T/66 has low water absorption, high heat resistance and excellent chemical resistance that widely used in aircraft tires, auto parts, power, electronics, communications, machinery, electric tools, sports goods manufacturing.
- Fully Aromatic Polyamides. For example, poly-p-phenylene terephthamide (PPTA, Fig 1. Compound 4) formed by the polycondensation reaction of p-phenylenediamine and terephthaloyl chloride, can be used in bulletproof products, cut-resistant products, friction sealing materials, etc.
- Others. Polyester amide contains both ester bond and amide bond, so it combines the characteristics of polyester and polyamide, showing excellent biodegradability, biocompatibility and mechanical properties. Polyester amide can be used in biomedicine, biological tissue engineering, thermoplastic elastomer and electronic intelligent materials.
Imide polymers are also known as polyimide (PI), which is a kind of polymer containing imide ring in the main chain. Based on excellent mechanical properties, heat resistance, dimensional stability, strong oxidation stability, chemical corrosion resistance such as acid and alkali, radiation resistance and good dielectric properties, PI has extremely high practical value and application fields, including aviation, electronics, electrical, automotive, precision instruments, household appliances, etc.
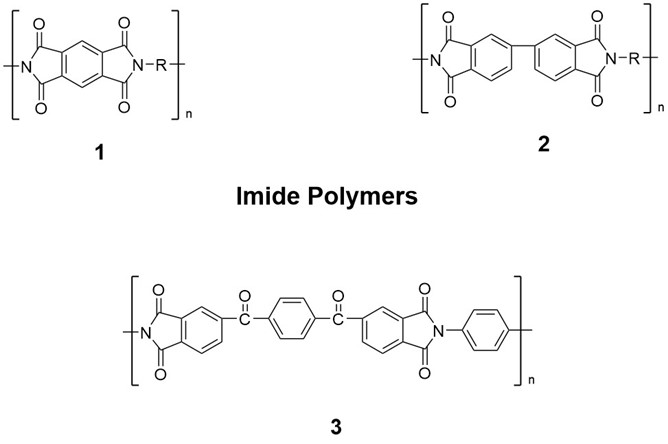 Fig. 2. Examples of imide polymers.
Fig. 2. Examples of imide polymers.
Currently, the PI materials widely used in industry are mainly benzene-type PI (Fig 2. Compound 1). However, biphenyl-type PI (Fig 2. Compound 2) exhibits better performance in special applications such as higher mechanical strength, better chemical resistance and liquid crystal effect, and low dielectric constant.[2] Other PIs are also in development. For example, Yang et al. prepared transparent thioether-type PI films.[3] Liu et al. used 4,4'-terephthaloyl diphthalic anhydride (TDPA) and p-phenylenediamine (PPD) as monomers to prepare a novel diketone anhydride-type PI by two-step method (Fig 2. Compound 3).[4]
If you are interested in our amide and imide polymers, please contact us immediately!
References
- Liang, K. Design, Preparation and Properties of Bio-based Cross-linked Polymers based on Amide Bonds. Beijing University of Chemical Technology. 2021.
- Liu, F.L. et al. Research and Application Progress of Biphenyl Polyimides. New Chemical Materials. 2014, 42(11): 202-205.
- Yang, C.P. et al. Colorless poly(ether-imide)s deriving from 2,2-bis[4-(3,4-dicarboxyphenoxy)phenyl]propane dianhydride (BPADA) and aromatic bis(ether amine)s bearing pendent trifluoromethyl groups. European Polymer Journal. 2005, 42(4): 721-732.
- Liu, Y.J. et al. Synthesis and properties of polyimides resistant to ultrahigh temperature diketone anhydride. Chinese Journal of Applied Chemistry. 2019, 36(06): 658-663.











 Fig. 1. Examples of amide polymers.
Fig. 1. Examples of amide polymers. Fig. 2. Examples of imide polymers.
Fig. 2. Examples of imide polymers.