Browse by
Introduction
Mercaptan refers to a class of compounds that contain a mercaptan functional group. Because of their strong binding to mercury compounds, they were originally used as mercury scavengers. Chemically, due to the combination of d orbitals and intrinsic electron density associated with sulfur, mercaptans are classified as soft nucleophiles compared to their alcohol and amine counterparts, and are generally associated with substrates that readily react with strong nucleophile, showing high reactivity. Mercaptans can play a crucial role in the facile synthesis of molecules in the fields of materials, optoelectronics, biology and engineering. Common types of mercaptan structures include alkyl mercaptans, mercaptan propionic acid mercaptans, thioacetic acid mercaptans, and aromatic mercaptans. The diagram below presents the structures of the various mercaptan, mercaptanate, and thioradical types.
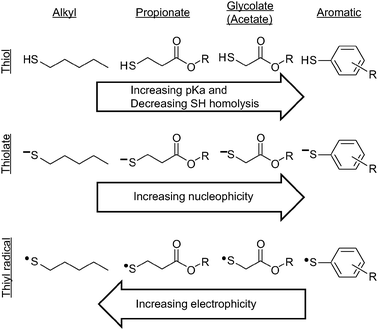 Fig. 1 Structures of various mercaptan, mercaptanate and thiyl radical types.[1]
Fig. 1 Structures of various mercaptan, mercaptanate and thiyl radical types.[1]
Mercaptan-based Chemical Reactions
Mercaptans possess the ability to react under mild conditions with a wide range of substrates such as electron-rich alkenes, alkynes, isocyanates, epoxy resins and halogens due to their high reactivity.
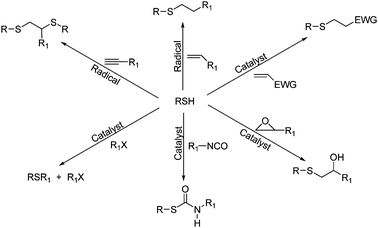 Fig. 2 Toolbox of mercaptan-click reactions.[1]
Fig. 2 Toolbox of mercaptan-click reactions.[1]
Mercaptan nucleophilic reactions are classified according to the nucleophilic attack of substrates on mercaptan or mercaptanate anions, including mercaptan-epoxy, mercaptan-isocyanate, mercaptan-halogen, mercaptan-Michael addition and other reaction types. Mercaptan-epoxy reactions have important applications in traditional synthesis, biology/pharmaceuticals, and industrial fields such as adhesives and composites. The mercaptan-isocyanate reaction has applications in high-performance coatings and optics. Mercaptans are typical soft nucleophiles that can undergo rapid and efficient substitution reactions with halogens with easily substituted leaving groups, and the resulting halide salts are easily removed in the presence of organic bases as precipitants . Finally, the base-mediated mercaptan-Michael reaction is a versatile and important synthetic tool that is widely used in polymer and organic chemistry, such as the preparation of α- and ω-functional (co)polymers.
There are two distinct radical-mediated mercaptan reactions, the mercaptan-ene and mercaptan-alkyne reactions. Both of these reactions achieve fast reaction rates and are powerful tools for the functionalization of small molecules, biomolecules, and polymers, which are widely used in electronic, optical, and biological fields. Like acrylic, styrene, and other radical-initiated polymerization and reaction processes, radical-mediated mercaptan-ene reactions involve three distinct reaction processes: initiation, polymerization or coupling, and termination. Most notably, the reaction is capable of self-initiating polymerization in the presence of UV light without providing any additional catalyst. The mercaptan-alkyne reaction is a complementary reaction to the mercaptan-alkene. The most important and remarkable feature of this reaction is the ability of the alkyne bond to react with two equivalents of mercaptans, i.e., the formation of bis-addition products with 1,2-regioselectivity. It also has all the advantages associated with the free radical mercaptan-ene reaction. Mercaptan-alkyne reactions can be used as a means to prepare tunable high-refractive-index thin films and as a tool for biomaterial synthesis.
If you are interested in our mercaptan monomer products, please contact us immediately!
Reference
- Hoyle C E, et al. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Cheminform. 2010, 39(4):1355-1387.


 Fig. 1 Structures of various mercaptan, mercaptanate and thiyl radical types.[1]
Fig. 1 Structures of various mercaptan, mercaptanate and thiyl radical types.[1] Fig. 2 Toolbox of mercaptan-click reactions.[1]
Fig. 2 Toolbox of mercaptan-click reactions.[1]











