Atom Transfer Radical Polymerization (ATRP)
Atom transfer radical polymerization (ATRP) is a successful example of controlled radical polymerization which involves equilibria between growing chain radicals and their dormant counterparts. It is different from conventional free radical polymerization because of the ability of a redox-active transition metal complex to affect the reversible activation/deactivation rate of the growth center. As the name implies, the atom transfer step is crucial in the whole polymerization procedure, which is responsible for predetermined molecular weights, narrow molecular weight distributions, and high degrees of chain end functionalities.
ATRP Mechanism
In an ATRP process, there are essentially three components --- initiator, catalyst, and monomer adding to solvent. The equilibrium equation has a high degree of complexity and is shown in Fig. 1. In general, the radicals, or the active species, are generated through a reversible redox process catalyzed by a transition metal complex (MtZ/L, composed of a transition metal MtZ with ligand L) which undergoes one-electron oxidation to a higher state (X-MtZ+1/L) with simultaneous abstraction of a (pseudo)halogen atom, X, from a dormant species, R-X. This reversible process is mediated with a rate constant of activation (kact) and deactivation (kdeact), and is on the left side most of the time, which minimizes the likelihood for two radicals to exist near each other at the same time and therefore minimizes termination. Polymer chains grow by the addition of the intermediate radicals to monomers in a manner similar to a conventional free radical polymerization, with the rate constant of propagation kp. Termination reactions (kt) also occur in ATRP, mainly through radical coupling and disproportionation.
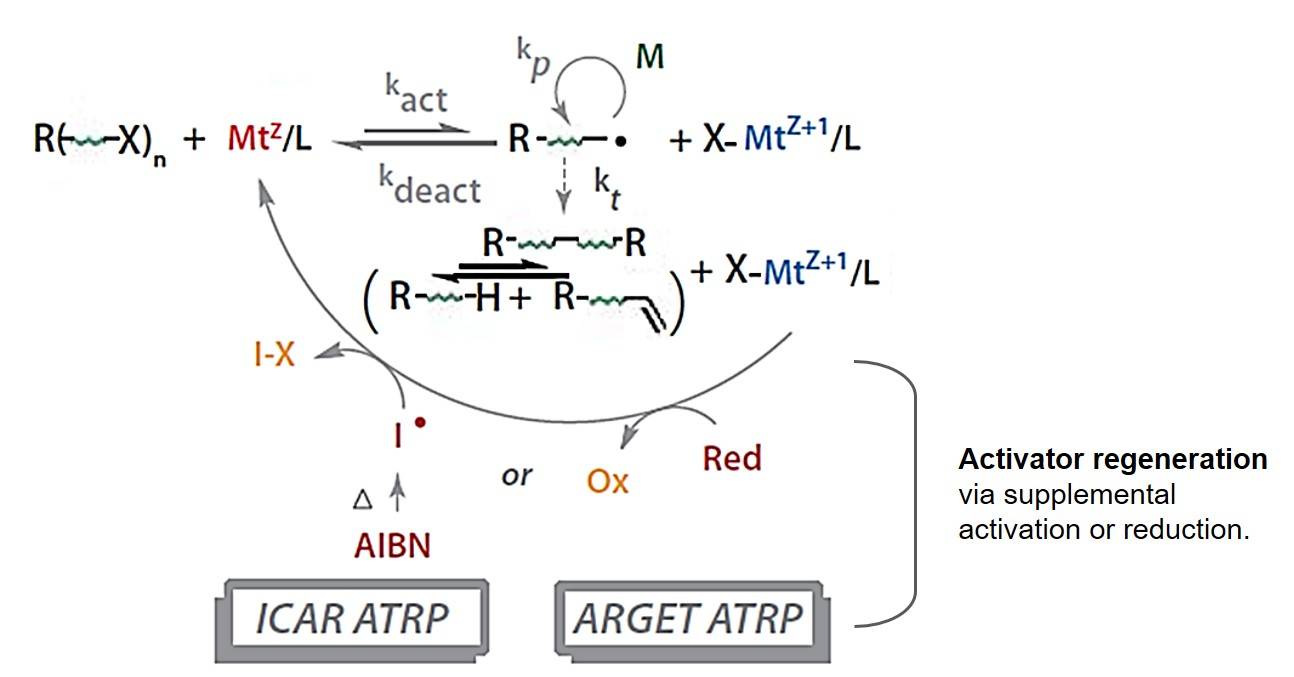 Fig. 1 Schematic illustration of the ATRP equilibrium.
Fig. 1 Schematic illustration of the ATRP equilibrium.
Components of ATRP
As a multi-component system, ATRP is composed of the monomer, an initiator with a transferable (pseudo) halogen, and a catalyst (composed of a transition metal species with any suitable ligand). Sometimes an additive is used. For a successful ATRP, other factors, such as solvent and temperature, must also be taken into consideration.
Monomer
A variety of monomers have been successfully polymerized using ATRP. Typical monomers are molecules containing substituents that can stabilize the propagating radicals, such as styrenes, (meth)acrylates, (meth)acrylamides, and acrylonitrile, etc. Each monomer possesses its own intrinsic radical propagation rate. Thus, for a specific monomer, the concentration of propagating radicals and the rate of radical deactivation need to be adjusted to maintain polymerization control.
Initiators
In ATRP, alkyl halides are typically used as the initiator (R-X) and the rate of the polymerization is first order with respect to the concentration of RX. In general, the efficiency increases with decreasing bond strength (R-Cl > R-Br > R-I). Thus, alkyl iodides are the most efficient initiators. However, alkyl iodides are light sensitive and tend to form metal iodide complexes. Therefore, bromo- and chloro-compounds are widely used. Typical ATRP initiators are 2-bromopropanitrile, ethyl 2-bromo-propionate, tosyl chloride among many other halides.
Catalyst & Ligand
The catalyst complex perhaps is most important among the components of ATRP. It determines the position of the atom transfer equilibrium and has an important influence on the polymerization rate. Generally, the transition metal center of the catalyst must have at least different oxidation states and should have a reasonable affinity toward a halogen. Various transition metals and ligands have been successfully employed in ATRP, but the most often used are the catalysts based on Cu (the two oxidation states are Cu(I) and Cu(II) and the nitrogen-based ligands, which are plotted along with the ratio of (kact/kdeact) in Fig. 2.
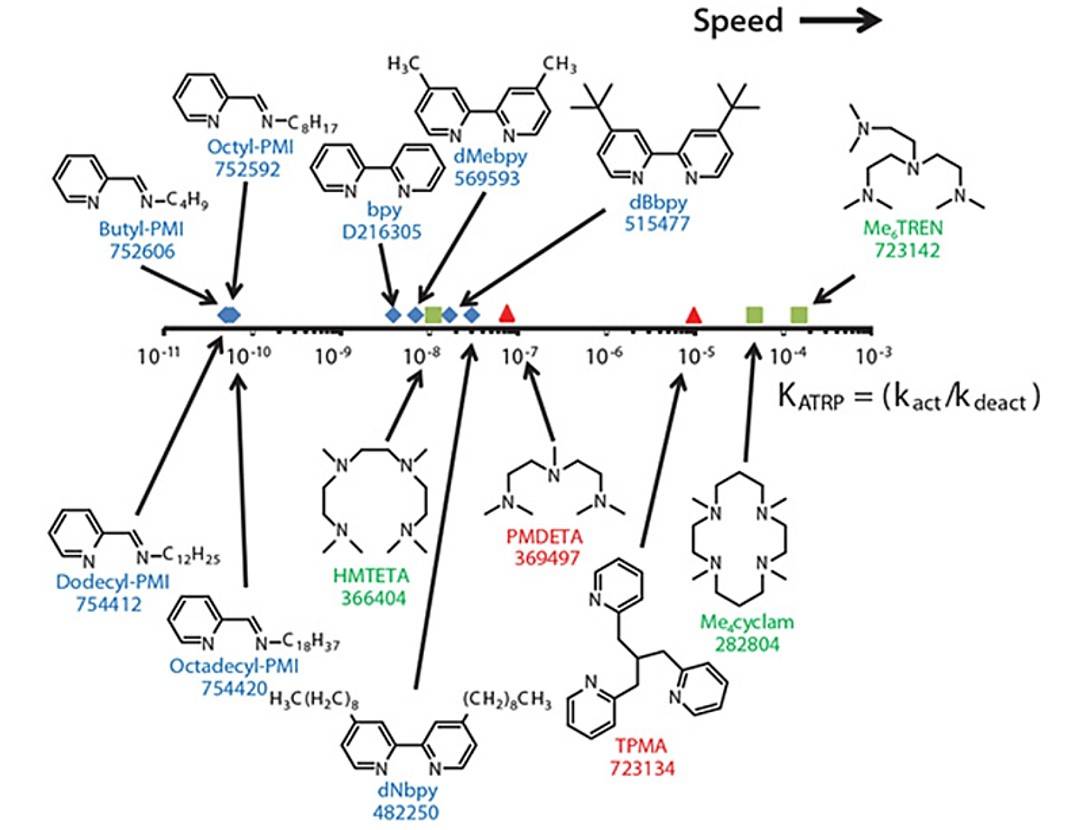 Fig. 2 Nitrogen-based ligands plotted against the ATRP equilibrium constant (KATRP) with initiator EtBibb, in the presence of CuBr in MeCN at 22 °C.
Fig. 2 Nitrogen-based ligands plotted against the ATRP equilibrium constant (KATRP) with initiator EtBibb, in the presence of CuBr in MeCN at 22 °C.
Solvent
ATRP can be carried out either in bulk, in solution, or in a heterogeneous system. Various solvents, such as benzene, toluene, anisole, 1,4-dioxane, ethyl acetate, acetone, dimethylformamide (DMF), dimethyl sulfoxide (DMSO), alcohol, water, and many others, have been used for different monomers. Several factors need to be considered when choosing a solvent, such as minimizing the possibility of chain transfer to solvent, minimizing the occurrence of solvent-assisted side reactions and controlling the interactions between solvent and catalytic system.
Variations of ATRP
Activator Regeneration ATRP
Since the catalyst complex is oxygen-sensitive, several variations of ATRP have been developed to make effort to minimize the handling of this compound. The progress of classical ATRP involves activator regeneration processes so that the air-sensitive catalyst can be generated at the start of the reaction and the loss of Cu(I) can be compensated, which is shown in Fig. 1.
- Initiators for Continuous Activator Regeneration (ICAR): this is a technique that uses a source of conventional radical initiators to continuously regenerate the Cu(I) activator, which would otherwise be consumed in termination reactions, when catalysts are used at very low concentrations.
- Activators Regenerated by Electron Transfer (ARGET): this technique employs non-radical forming reducing agents, such as ascorbic acid, sugars, phenol, glucose, hydrazine, tin(II)2-ethyl hexanoate (Sn(EH)2), and Cu0. etc., to react with Cu(II) for regeneration of Cu(I).
Other ATRP Processes
- Reverse ATRP: this is a technique by adding the catalyst complex in its higher oxidation state, which was then converted to the activator by reacting with a standard free radical initiator (e.g., AIBN).
- Metal-free ATRP: the residual metal catalyst in the final product always limits the application of ATRP in biomedicine and electronics. Metal-free ATRP is a novel method developed in 2014 involving a photo-redox reaction of 10-phenothiazine. This technique has been demonstrated to be capable of polymerization of methacrylates and acrylonitrile.
- Mechano/sono-ATRP: this method involves mechanical forces, typically ultrasonic agitation, as an external stimulus to induce the generation of activators in ATRP.
Applications
Compared with ionic polymerization, ATRP has the advantages of wide monomer coverage, mild polymerization conditions, and easy to scale up. Using the ATRP technique, a wide variety of polymers with different topological structures, compositions and functionalized structures can be prepared. They have broad applications in high-performance adhesives, dispersants, surfactants, polymer alloy solubilizers and processing aids, thermoplastic elastomers, green chemicals, electronic materials and new fluorine-containing materials.
- Random, alternating and ladder copolymers
- Block copolymers
- Grafting and comb copolymers
- Polymer brush
- Hyperbranched, star polymers
- Functionalized polymers used for drug delivery
- Organic-inorganic hybrid materials

 Fig. 1 Schematic illustration of the ATRP equilibrium.
Fig. 1 Schematic illustration of the ATRP equilibrium. Fig. 2 Nitrogen-based ligands plotted against the ATRP equilibrium constant (KATRP) with initiator EtBibb, in the presence of CuBr in MeCN at 22 °C.
Fig. 2 Nitrogen-based ligands plotted against the ATRP equilibrium constant (KATRP) with initiator EtBibb, in the presence of CuBr in MeCN at 22 °C.


