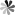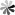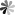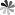Polymerization Inhibitors
- CAS: 128-39-2
- Molecular Weight: 206.32
- Molecular Formula: C14H22O
- CAS: 13927-71-4
- Molecular Weight: 472.34
- Molecular Formula: C18H36 Cu N2 S4
- CAS: 1879-09-0
- Molecular Weight: 178.27
- Molecular Formula: C12H18O
- CAS: 1898-66-4
- Molecular Weight: 394.32
- Molecular Formula: C18H12N5O6 *
- CAS: 1948-33-0
- Molecular Weight: 166.22
- Molecular Formula: C10H14O2
- CAS: 24806-57-3
- Molecular Weight: 268.08
- Molecular Formula: C10H13BF4OS
- CAS: 3584-23-4
- Molecular Weight: 421.92100
- Molecular Formula: C12H7Cl6N3O
- CAS: 3602-55-9
- Molecular Weight: 164.20
- Molecular Formula: C10H12O2
- CAS: 98-29-3
- Molecular Weight: 166.22
- Molecular Formula: C10H14O2
Introduction

Polymerization inhibitors are substances that are added to a monomer to prevent spontaneous polymerization. In order to avoid the self-polymerization and copolymerization of monomers during storage and transportation, effective polymerization inhibitors are often added to the monomers. The polymerization inhibitor can prevent the polymerization from proceeding and the induction period (a period of time during which the polymerization rate is zero) is produced in the polymerization process. The polymerization inhibitors commonly applied in the industry are phenolic, amines and free radical-nitroxide compounds.
Types of Polymerization Inhibitors
Phenolic compounds, such as polyphenols and substituted phenols, are widely used and effective inhibitors for olefin separation. As shown in Fig 1, the polymerization inhibition of phenolic compounds is achieved through the hydrogen transfer reaction between phenolic compounds and free radicals to generate conjugated radicals. In addition, when oxygen is involved in the reaction system, the synergistic effect of phenolic compounds and oxygen leads to enhanced polymerization inhibition activity. For example, the free groups of hydroquinone and its derivatives react with oxygen to form peroxy free groups, which react with hydroquinone to form free group complexes, and finally obtain stable polymers.
 Fig. 1 Structure of inhibitory phenolic compounds (Journal of Molecular Liquids. 2022, 348: 118387).
Fig. 1 Structure of inhibitory phenolic compounds (Journal of Molecular Liquids. 2022, 348: 118387).
Like phenolic compounds, amines are also synergistic with oxygen, showing better resistance to polymerization under oxygen conditions. As shown in Fig 2, the anti-polymerization effect of amines is also achieved through the hydrogen transfer reaction between amines and chain radicals. The most commonly used hydroxylamine inhibitor in industry is diethylhydroxylamine, which is volatile, easily soluble in water, less toxic to humans, and insensitive to temperature.
 Fig. 2 Structure of inhibitory amines compounds (In Industrial Chemistry Library. 1996, 8: 489-505).
Fig. 2 Structure of inhibitory amines compounds (In Industrial Chemistry Library. 1996, 8: 489-505).
- Free Fadical-nitroxide Compounds
As shown in Fig 3, free radicals-nitroxide radicals generate stable inactive molecules by decomposing peroxides, which can effectively capture active chain free radicals and enhance the anti-polymerization effect. Meanwhile, the introduction of oxygen can significantly increase the activity of nitric acid compounds, thereby inhibiting their activity.
 Fig. 3 Structure of inhibitory free radical-nitroxide compounds (Polymer Chemistry. 2018, 9(13): 1479-1516).
Fig. 3 Structure of inhibitory free radical-nitroxide compounds (Polymer Chemistry. 2018, 9(13): 1479-1516).
If you are interested in our polymerization inhibitors, please contact us immediately!
References
- Verma, C. et al. Aqueous phase polymeric corrosion inhibitors: Recent advancements and future opportunities. Journal of Molecular Liquids. 2022, 348: 118387.
- Lartigue, F. The use of phenolic compounds as free-radical polymerization inhibitors. In Industrial Chemistry Library. 1996, 8: 489-505.
- Hansen, K.A. et al. Nitroxide radical polymers-a versatile material class for high-tech applications. Polymer Chemistry. 2018, 9(13): 1479-1516.












 Fig. 1 Structure of inhibitory phenolic compounds (Journal of Molecular Liquids. 2022, 348: 118387).
Fig. 1 Structure of inhibitory phenolic compounds (Journal of Molecular Liquids. 2022, 348: 118387). Fig. 2 Structure of inhibitory amines compounds (In Industrial Chemistry Library. 1996, 8: 489-505).
Fig. 2 Structure of inhibitory amines compounds (In Industrial Chemistry Library. 1996, 8: 489-505). Fig. 3 Structure of inhibitory free radical-nitroxide compounds (Polymer Chemistry. 2018, 9(13): 1479-1516).
Fig. 3 Structure of inhibitory free radical-nitroxide compounds (Polymer Chemistry. 2018, 9(13): 1479-1516).