Browse by
Introduction
The properties, forms and uses of acrylic acid and its ester polymers vary by monomer and polymerization method. Currently, acrylic polymers are widely used in daily chemicals, textiles, coatings, food, construction, adhesives, medical treatment, water treatment and electronics and other fields. With the continuous deepening of industrial manufacturing and production research and development, the demand for acrylic acid varieties in various industries has also increased rapidly. Among the various applications of acrylic, acrylate emulsions and solution polymers are the most widely used. Acrylate emulsion polymers are widely used in various coating formulations, chemical fiber filling, non-woven fabrics, fabric bonding or lamination, cashmere, back coating and printing pigment base materials due to their excellent transparency, hardness, color durability, UV stability and chemical inertness. In addition, acrylate emulsions can also be used as processing agents for polyvinyl chloride (PVC) materials to improve toughness, impact resistance, etc.
Generally, acrylic polymers can be divided into acrylate & methacrylate polymers and acrylic acid polymers. Among them, acrylate copolymer (AP) is a general term for polymers obtained by copolymerization of acrylate, including methyl acrylate, ethyl ester, butyl ester and methyl methacrylate. Acrylate monomers can be additional acrylic compounds or other unsaturated compounds with double bonds, because the monomers have living double bonds and are prone to self-polymerization as well as copolymerization (Fig. 1).
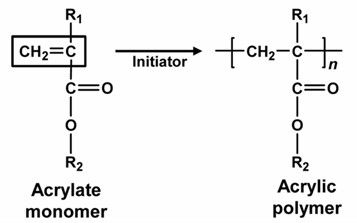 Fig. 1. Acrylic polymer synthesis.
Fig. 1. Acrylic polymer synthesis.
Direct esterification of alcohols and acrylic acid is the only important way to produce acrylates. Macromonomers can produce 40 or 50 different products, mainly including methyl acrylate (MA), ethyl acrylate (EA), butyl acrylate (n-BA), acrylic acid-2-7, ester and acrylic acid, among others. The yield accounts for more than 95% of the yield of acrylic monomer series. Since Joseph Redenbach first discovered the oxidation of acrolein to acrylic acid in 1843, its production methods have successively experienced fluoroethanol method, oxo synthesis method, ketene method, acrylonitrile hydrolysis method and propylene oxidation method. But due to technical and economic reasons, these four processes have been basically eliminated. Presently, almost all acrylic acids and esters in industrial production use propylene two-step oxidation technology.
If you are interested in our acrylic polymers, please contact us immediately!
References
- Sennakesavan, G. et al. Acrylic acid/acrylamide based hydrogels and its properties - A review. Polymer Degradation and Stability. 2020: 109308.
- Pratap, A. et al. Vinyl ester and acrylic based polymer concrete for electrical applications. Progress in Crystal Growth & Characterization of Materials. 2002, 45(1/2): 117-125.
- Nigro, V. et al. Chemical-Physical Behaviour of Microgels Made of Interpenetrating Polymer Networks of PNIPAM and Poly(acrylic Acid). Polymers. 2021: (9).
- Gaytán, et al. Current status on the biodegradability of acrylic polymers: microorganisms, enzymes and metabolic pathways involved. Applied Microbiology and Biotechnology. 2021, 105(3): 991-1006.
- Eckersley, S.T. et al. Drying behavior of acrylic latexes. Progress in Organic Coatings. 1994, 23(4): 387-402.


 Acrylate & Methacrylate Polymers
Acrylate & Methacrylate Polymers
 Acrylic acid Polymers
Acrylic acid Polymers
 Fig. 1. Acrylic polymer synthesis.
Fig. 1. Acrylic polymer synthesis.