Browse by
Introduction
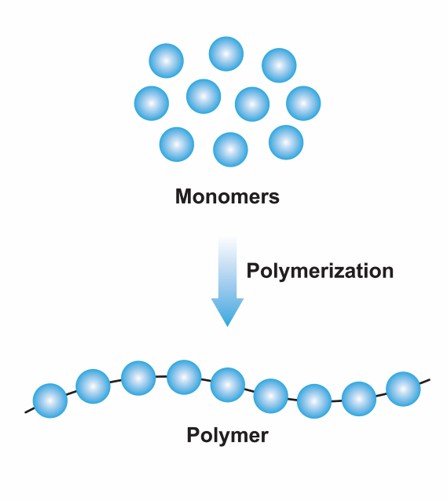
The synthetic blocks or repeating units in a polymer are called monomers. Polymerization or polymer synthesis is a chemical reaction in which monomers are covalently bonded together to form a polymer structure. The nature of the monomer is crucial to the properties of the final polymer. For example, the monomer of polyethylene is ethylene, which has no rigid large groups in its molecular structure. The monomer of polystyrene is styrene containing benzene rings. Ultimately, polyethylene is weaker than polystyrene in mechanical properties, heat resistance, etc. Depending on the polymerization reaction, monomers can be divided into two categories: addition polymerization monomers and condensation polymerization monomers. Addition polymerization monomers are monomer molecules with double or triple bonds or ring structures, which can be used to produce polymers by addition polymerization. For example, the C=C bond of ethylene can be opened for polymerization to produce polyethylene. If two or more monomer molecules containing functional groups can react with each other to form water or other separable small molecules, the monomers that can undergo polycondensation reaction to form polymers are called polycondensation monomers. For example, the monomers of PET are bisphenol A and phosgene.
Functionality of Monomers
The number of functional groups in a monomer that can participate in a reaction is the functionality. Monomers with a functional degree of 1 can only form dimers, monomers with a functional degree of 2 can form linear polymers, and molecules with a functional degree greater than 2 can form bulk polymers. Typically, bulk polymers have a higher strength than linear polymers. The nature of the monomer has an important influence on the properties of the final polymer, so we can choose the right monomer or modify it to obtain the polymer we want.
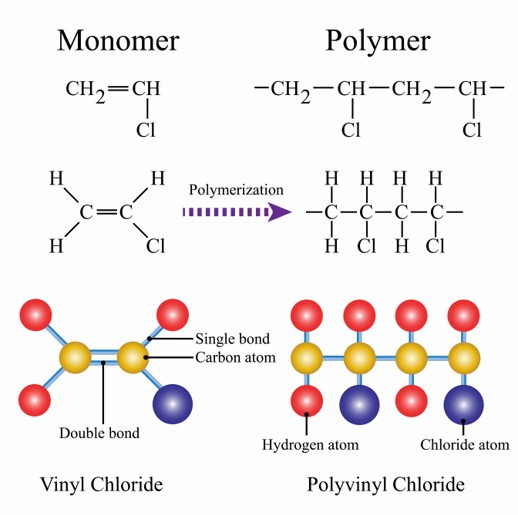
The Role of Monomers in Polymers
Usually, the variable physicochemical properties of the monomers also confer variable properties to the synthetic polymers. For example, polyethylene synthesized from ethylene and polypropylene synthesized from propylene, both of which have significant differences in mechanical properties, weather resistance, and low temperature resistance. When monomers contain a large number of structures such as benzene rings, the heat resistance of the final synthesized polymers, such as polyimides, will be greatly improved. Therefore, we can choose monomers with different properties to synthesize out polymers to better meet the usage requirements.
If you are interested in our monomer products, please contact us immediately!


 Acrylic Monomers
Acrylic Monomers
 Alcohol Monomers
Alcohol Monomers
 Allyl Monomers
Allyl Monomers
 Amide & Imide Monomers
Amide & Imide Monomers
 Amine Monomers
Amine Monomers
 Anhydride Monomers
Anhydride Monomers
 Biodegradable Polymer Monomers
Biodegradable Polymer Monomers
 Carboxylic Acid Monomers
Carboxylic Acid Monomers
 Cycloolefin
Cycloolefin
 Dendrimer Building Blocks
Dendrimer Building Blocks
 Epoxide Monomers
Epoxide Monomers
 Halide Monomers
Halide Monomers
 Isocyanate Monomers
Isocyanate Monomers
 Mercaptan Monomers
Mercaptan Monomers
 Other Monomers
Other Monomers
 Styrenic Monomers
Styrenic Monomers
 Vinyl Monomers
Vinyl Monomers