Nitroxide-mediated Radical Polymerization (NMP)
Nitroxide-mediated radical polymerization (NMP) is a controlled radical polymerization (CRP) technique that relies on the reversible capture of the propagating species by nitroxides with the formation of dormant chains (alkoxyamines). It is very attractive because of its metal-free property and effectiveness in the polymerization of a broad range of monomers with various functionalities. Its living character has opened up enormous possibilities for polymers with well controlled stereochemistry, very low dispersity, as well as predictable molecular weight and composition in a wide range of applications.
Mechanism
NMP can be conducted either through a unimolecular process or a bimolecular process.
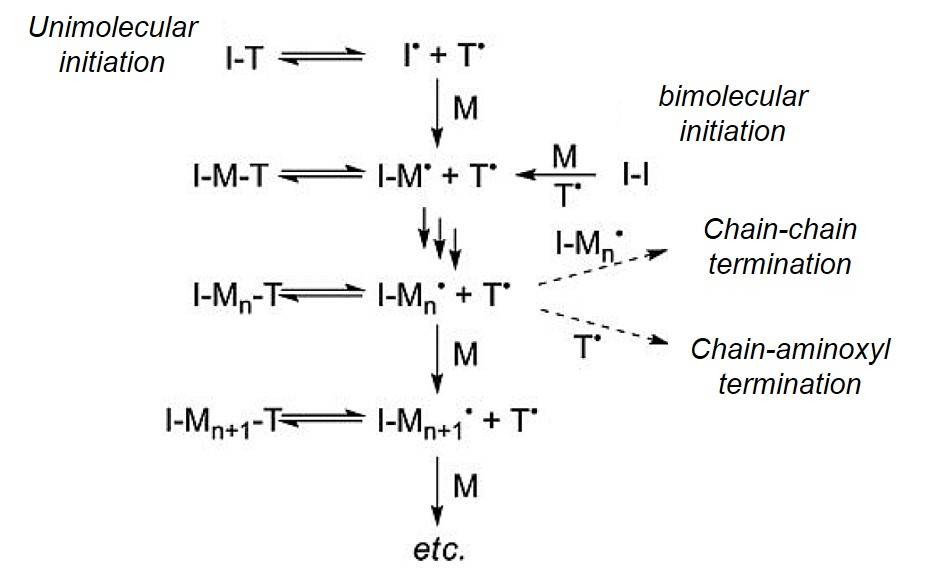 Fig. 1 General illustration of NMP mechanism. (Polymer Reviews, 2011, 51(2): 104-137)
Fig. 1 General illustration of NMP mechanism. (Polymer Reviews, 2011, 51(2): 104-137)
- Unimolecular Process
Referring to the concept of well-defined initiators from living anionic and cationic procedures, unimolecular initiators for NMP have been developed. Both desired functional groups and nitroxide radicals are on the active species so that the initiators can be used as unimolecular agents based on the alkoxyamine functionality. The C-O bond of the small molecule alkoxyamine derivative is therefore expected to be thermally labile and decompose on heating to give an initiating radical and stable mediating nitroxide radical, i.e., the α-methylbenzyl radical as well as the mediating nitroxide radical in the correct 1:1 stoichiometry.
- Bimolecular Process
In the bimolecular process, NMP involves a combination of radical initiator (Ι), monomer (M) and nitroxide radical (R), for trapping intermediate radical species. Initiator and nitroxide radicals are separately provided by two substances. Radicals produced by benzoyl peroxide (BPO) via thermolysis are capable of initiating the polymerization. At the same time, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) is employed to trap a styrenyl radical to form a thermally labile alkoxyamine that controls the chain termination and chain transfer reactions. This process is a temporary inactivation and when the temperature rises, the dormant species can generate reactive chain radicals again to continue the propagation proceeds. It is important that the stable nitroxide radicals are capable of reversible termination reactions, but do not initiate polymerizations.
Alkoxyamines Initiators for NMP
The initiating materials that work on the NMP process are a family of compounds referred to as alkoxyamines, providing both the reactive initiating radical and the stable mediating nitroxide radical. The development of stable, readily functionalized alkoxyamines initiators, which mimic the growing chain end for living free radical systems, is a crucial process in NMP. To date, a number of synthetic routes to alkoxyamines have been developed. As a rule, they mainly rely on the generation of carbon-centered radicals that are trapped by a preformed nitroxide.
a. Initially, the synthetic approaches to alkoxyamines involving the reaction of BPO with an excess of styrene to give a benzylic radical followed by trapping of the radical intermediate with TEMPO, as shown in Fig. 2.
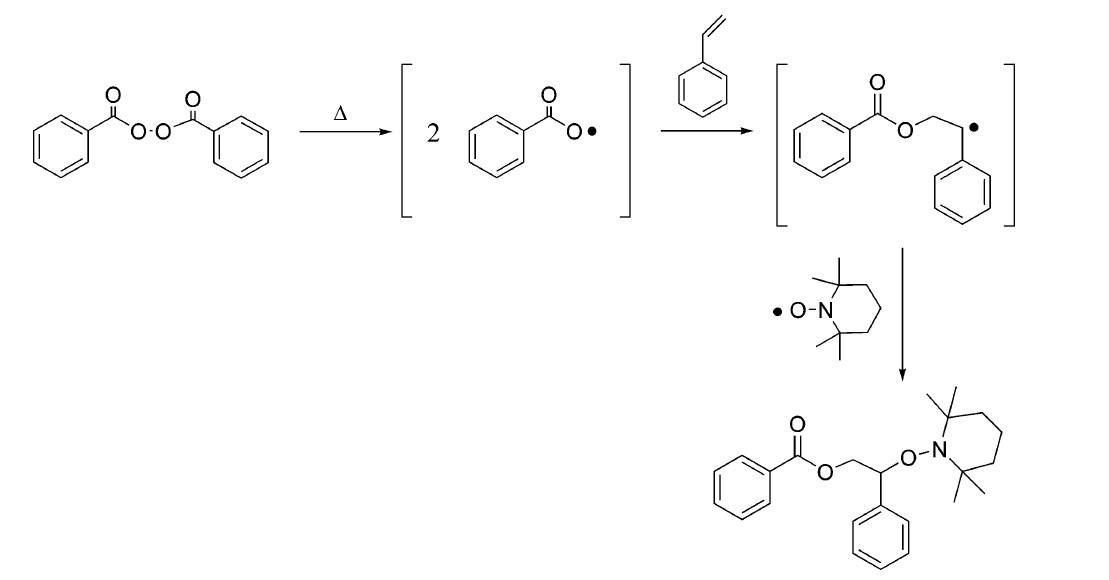 Fig. 2 Approach to alkoxyamines involving BPO, TEMPO and styrene monomer. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 2 Approach to alkoxyamines involving BPO, TEMPO and styrene monomer. (Chemical reviews, 2008, 108(3): 1104-1126)
b. In addition, alkoxyamines could also be prepared by thermal decomposition of azo-initiators, i.e., azobisisobutyronitrile (AIBN), in the presence of a nitroxide.
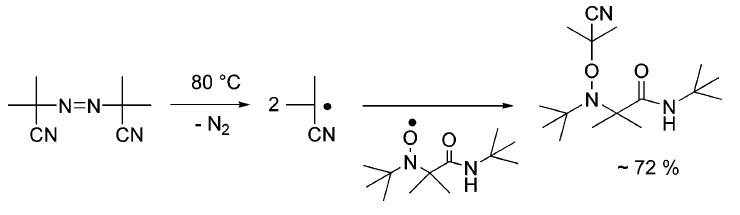 Fig. 3 Approach to alkoxyamines through azo-initiators. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 3 Approach to alkoxyamines through azo-initiators. (Chemical reviews, 2008, 108(3): 1104-1126)
c. Alternative preparation approach to alkoxyamines could be conducted by organic halides and a nitroxide (TEMPO) in the presence of a copper complex.
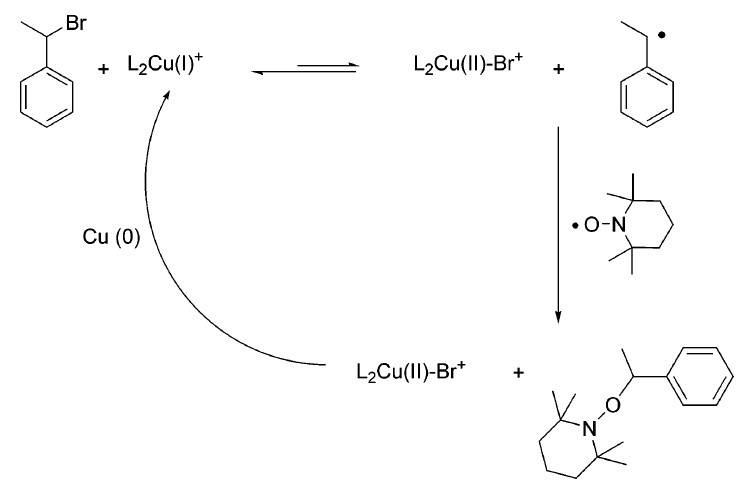 Fig. 4 Approach to alkoxyamines in the presence of a copper complex. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 4 Approach to alkoxyamines in the presence of a copper complex. (Chemical reviews, 2008, 108(3): 1104-1126)
d. Finally, polyfunctional alkoxyamines (di- or tri-alkoxyamines), could be prepared on a basis of DEPN and poly-alkyl halides by atom transfer radical addition.
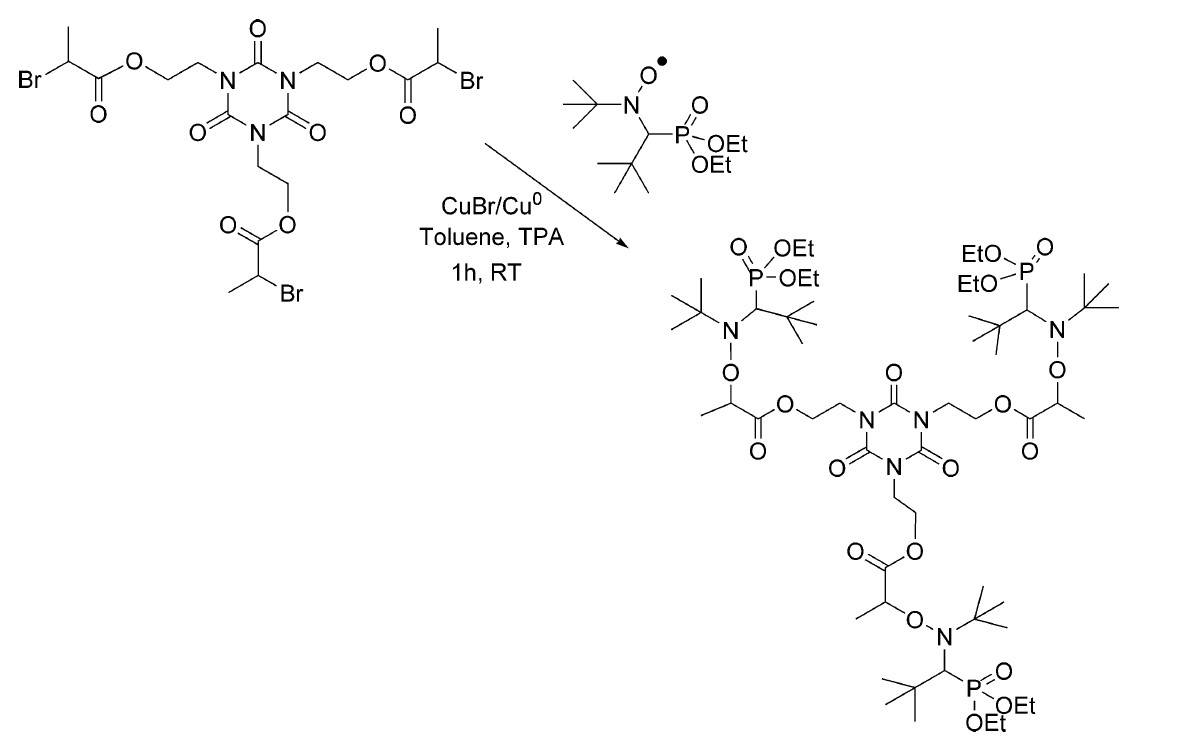 Fig. 5 Approach to polyfunctional alkoxyamines. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 5 Approach to polyfunctional alkoxyamines. (Chemical reviews, 2008, 108(3): 1104-1126)
Applications
NMP offers clear advantages in allowing the polymerization of a broad range of functional monomers (excepting methacrylates) into a range of random, gradient, block, and branched copolymer architectures and it has proven to be readily adapted to industrially relevant methods including polymerization in dispersed systems and continuous reactors.
- Synthesis of copolymers
a. Statistical/gradient copolymers
b. Block copolymers
c. Graft, star, and branched copolymers
- Surface growth of polymers and copolymers
NMP is a typical controlled radical polymerization method for surface modification via the attachment of alkoxyamines with subsequent polymer growth from the surface.
- Easy for post-polymerization modification of polymers
a. Replacement of nitroxide radicals
Using maleic anhydride or maleimide derivatives to react with the alkoxyamine at the end of the polymer, then eliminate the generated nitroxide radicals can introduce polycyclic aromatics and other various functional groups at the end of the polymer.
 Fig. 6 Synthesis of functionally terminated polymer via NMP followed by a reduction of the alkoxyamine
Fig. 6 Synthesis of functionally terminated polymer via NMP followed by a reduction of the alkoxyamine
b. Chain-end functionalization through nitroxide exchange
Since the thermally-labile alkoxyamine unit present in terminal chains after polymerization, nitroxide exchange reactions could be used to prepare coupled homopolymers, star-like microgels, reversibly cross-linked gels, and coupled zeolite crystals under heating conditions.
 Fig. 7 Synthesis of functionally terminated polymer via NMP followed by a nitroxide exchange.
Fig. 7 Synthesis of functionally terminated polymer via NMP followed by a nitroxide exchange.
References:
- Hawker, C. J.; Bosman, A. W., et.al. New polymer synthesis by nitroxide mediated living radical polymerizations, Chem. Rev. 2001, 101, 3661–3688.
- Grubbs R B. Nitroxide-mediated radical polymerization: limitations and versatility. Polymer Reviews, 2011, 51(2): 104-137.
- Sciannamea V, Jérôme R, et.al. In-situ nitroxide-mediated radical polymerization (NMP) processes: their understanding and optimization. Chemical reviews, 2008, 108(3): 1104-1126.

 Fig. 1 General illustration of NMP mechanism. (Polymer Reviews, 2011, 51(2): 104-137)
Fig. 1 General illustration of NMP mechanism. (Polymer Reviews, 2011, 51(2): 104-137) Fig. 2 Approach to alkoxyamines involving BPO, TEMPO and styrene monomer. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 2 Approach to alkoxyamines involving BPO, TEMPO and styrene monomer. (Chemical reviews, 2008, 108(3): 1104-1126) Fig. 3 Approach to alkoxyamines through azo-initiators. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 3 Approach to alkoxyamines through azo-initiators. (Chemical reviews, 2008, 108(3): 1104-1126) Fig. 4 Approach to alkoxyamines in the presence of a copper complex. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 4 Approach to alkoxyamines in the presence of a copper complex. (Chemical reviews, 2008, 108(3): 1104-1126) Fig. 5 Approach to polyfunctional alkoxyamines. (Chemical reviews, 2008, 108(3): 1104-1126)
Fig. 5 Approach to polyfunctional alkoxyamines. (Chemical reviews, 2008, 108(3): 1104-1126) Fig. 6 Synthesis of functionally terminated polymer via NMP followed by a reduction of the alkoxyamine
Fig. 6 Synthesis of functionally terminated polymer via NMP followed by a reduction of the alkoxyamine Fig. 7 Synthesis of functionally terminated polymer via NMP followed by a nitroxide exchange.
Fig. 7 Synthesis of functionally terminated polymer via NMP followed by a nitroxide exchange.


