Browse by
Introduction
Polymer is a long-chain molecule or macromolecule built by linking repeating chemical units. Each polymer molecule can consist of hundreds, thousands or even millions of repeating units. The IUPAC Gold Book defines a macromolecule as a molecule of high relative molecular mass whose structure consists essentially of multiple repeating units derived, actually or conceptually, from molecules of low relative molecular mass.
Units in polymers are usually held together by covalent bonds. Adjacent polymer chains or different parts of the same chain can be held together by intermolecular forces or van der Waals bonds. In some cases, ionic bonds may also occur in polymers. Covalent bonds are characterized by relatively high energies, fixed angles, and short distances (0.11-0.16 nm), which determine the mechanical, thermal, chemical, and photochemical properties of polymers. According to the classification of monomers (Fig. 1), polymers can be divided into homopolymer and copolymer. Copolymers including alternating copolymer, random copolymer, and block copolymer. According to the shape of the chain, polymers can be classified into linear polymer, branched polymer, network polymer, star polymer, dendrimer, etc.
 Fig. 1. The classification of polymers.
Fig. 1. The classification of polymers.
Polymers include natural polymer and synthetic polymer. Organic natural polymers play a crucial role in biology, providing basic structural materials and participating in vital life processes. For example, the solid parts of all plants are polymerized from cellulose, lignin and various resins. Other important natural polymers include proteins, nucleic acids starches. Many inorganic polymers also are found in nature, including diamond and graphite. Both are composed of carbon.
Applications
As chemicals, the main applications of synthetic polymers are as follows:
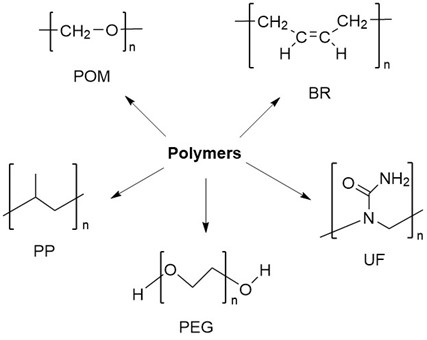 Fig. 2. Different polymers.
Fig. 2. Different polymers.
Currently, a variety of polymer chemicals have been used in the synthesis and production of general-purpose plastics, including polyethylene, polypropylene, polyvinyl chloride, styrene resins, phenolic and amino resins, epoxy resins, and polyurethanes. Among them, polypropylene (PP) is widely used in the textile industry and the manufacture of molded products due to its crystalline and thermoplastic properties.
Polyamide, polycarbonate, polyoxymethylene, polyphenylene ether, polyester resin, polyimide, polyphenylene sulfide, polysulfone, polyaryl ether ketone and other polymers are widely used in engineering plastics manufacturing industry.
Polymer chemicals can also be widely developed as synthetic raw materials of rubber, such as general rubber, special rubber, thermoplastic elastomer, etc. For example, cis-polybutadiene (BR), which is polymerized from butadiene, has excellent cold resistance, abrasion resistance, and elasticity compared to other general-purpose rubbers.
Polymer chemicals can also be used to synthesize viscose fiber, polyester fiber, polyamide fiber, polyacrylonitrile fiber, etc. For example, polyurethane (PU) is used in the manufacture of elastomeric fibres, coating bases and flexible/rigid foams.
Polymers are also the raw materials for the manufacture of various polymeric biomaterials. For example, polyethylene glycol (PEG) is widely used in various pharmaceutical formulations including injectable, topical, ophthalmic, oral, and rectal formulations due to its broad water solubility, non-volatile, physiologically inert, and lubricity properties agent. In addition, polymer products are also used to produce adhesives for textiles and paper and various paint coatings.
If you are interested in our polymer standards, please contact us immediately!
Reference
- Jialanella, G.L. 9-Advances in bonding plastics, Advances in Structural Adhesive Bonding. Woodhead Publishing in Materials. 2010, 237-264.


 Poly(styrene sulfonate sodium) Standards
Poly(styrene sulfonate sodium) Standards
 Polystyrene Standards
Polystyrene Standards
 Fig. 1. The classification of polymers.
Fig. 1. The classification of polymers. Fig. 2. Different polymers.
Fig. 2. Different polymers.









