Browse by
Introduction
Amines are organic compounds containing nitrogen atoms and lone pairs of atoms. Basically, they are derived from ammonia (NH3) where one or more hydrogen atoms are replaced by alkyl or aryl groups, hence they are called fatty amine monomer and aromatic amine monomer, respectively. In addition, amines can also be divided into primary amines, secondary amines and tertiary amines according to the nature and number of substituents on nitrogen. Next, the properties and applications of several representative amine compounds will be introduced in detail.
Fatty Amine Monomers
Ethylenediamine (EDA) is a colorless or slightly yellow oily or watery transparent liquid with the chemical formula C2H8N2. Ethylenediamine is a typical aliphatic diamine and is an alkaline substance. Global ethylenediamine production is mainly concentrated in the United States, Western Europe, Japan and other large companies, whose production capacity accounts for about 90% of the world's total production capacity. Ethylenediamine can be used in the manufacture of fuels, rubber vulcanization accelerators, pharmaceuticals and intermediates in the production of insulating varnishes. In particular, highly uniform and very large diameter PU microspheres were obtained by adding EDA (Fig. 1), which can be used in the fields of enzyme immobilization, catalytic reactor, adsorption and removal of dyes and metal ions, etc.
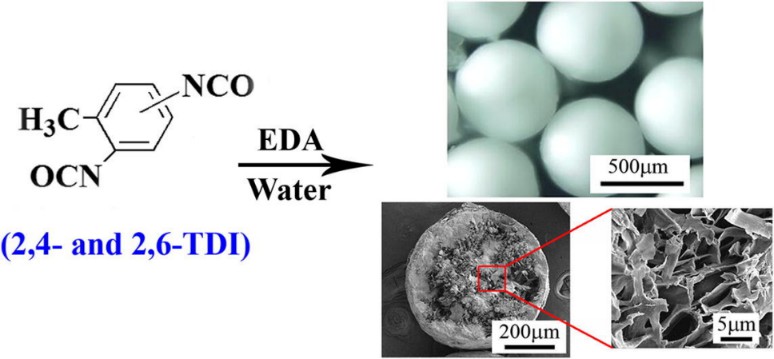 Fig. 1. Preparation of highly uniform PU microspheres by EDA (Chemical Engineering Journal. 2016, 303: 48-55).
Fig. 1. Preparation of highly uniform PU microspheres by EDA (Chemical Engineering Journal. 2016, 303: 48-55).
Hexamethylene diamine is an organic compound with a molecular formula of C6H16N2, which has a N-hexane carbon chain skeleton and amino functional groups at both ends. Hexamethylenediamine is a colorless solid with a strong ammonia odor. Hexamethylenediamine is widely used in the production of polyamides and diisocyanates, as well as curing agents for urea-formaldehyde resins, epoxy resins, and organic cross-linking agents. For example, a series of polyurethanes with different tensile strength and elongation at break, thermal stability and glass transition temperature were obtained by adding hexamethylene diamine (Fig. 2).
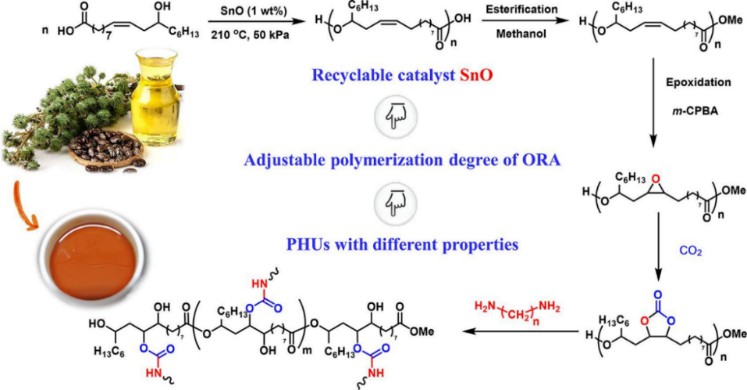 Fig. 2. Preparation of polyurethanes by hexamethylene diamine (European Polymer Journal. 2021, 153: 110501).
Fig. 2. Preparation of polyurethanes by hexamethylene diamine (European Polymer Journal. 2021, 153: 110501).
Aromatic Amine Monomers
Aniline (C6H7N), also known as aminobenzene, is one of the most important amines. Aniline molecule is a colorless oily liquid, slightly soluble in water, and easily soluble in organic solvents such as ethanol and ether. Aniline is widely used in the manufacture of dyes, drugs and resins, and can also be used as a rubber vulcanization accelerator. For example, the copolymerization of aniline and gallic acid can prepare a novel electroactive material with antioxidant and antibacterial activities (Fig. 3). Importantly, its derivative methyl orange can be used as an indicator for acid-based titrations. But aniline itself is harmful to the environment and pollutes the water.
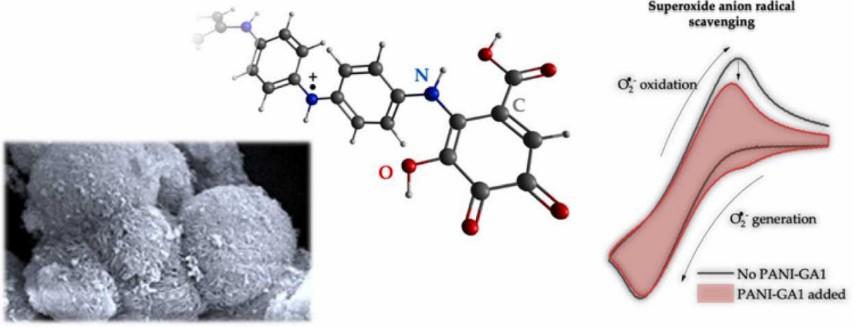 Fig. 3. Preparation of a novel electroactive material (Synthetic Metals. 2022, 286: 117048).
Fig. 3. Preparation of a novel electroactive material (Synthetic Metals. 2022, 286: 117048).
If you are interested in our amine monomers, please contact us immediately!
References
- Zhang, F. et al. Preparation of uniform and porous polyurea microspheres of large size through interfacial polymerization of toluene diisocyanate in water solution of ethylene diamine. Chemical Engineering Journal. 2016, 303: 48-55.
- Ren, F.Y. et al. Oligomeric ricinoleic acid synthesis with a recyclable catalyst and application to preparing non-isocyanate polyhydroxyurethane. European Polymer Journal. 2021, 153: 110501.
- Janošević, A. et al. Copolymerization of aniline and gallic acid: Novel electroactive materials with antioxidant and antimicrobial activities. Synthetic Metals. 2022, 286: 117048.



 Fig. 1. Preparation of highly uniform PU microspheres by EDA (Chemical Engineering Journal. 2016, 303: 48-55).
Fig. 1. Preparation of highly uniform PU microspheres by EDA (Chemical Engineering Journal. 2016, 303: 48-55). Fig. 2. Preparation of polyurethanes by hexamethylene diamine (European Polymer Journal. 2021, 153: 110501).
Fig. 2. Preparation of polyurethanes by hexamethylene diamine (European Polymer Journal. 2021, 153: 110501). Fig. 3. Preparation of a novel electroactive material (Synthetic Metals. 2022, 286: 117048).
Fig. 3. Preparation of a novel electroactive material (Synthetic Metals. 2022, 286: 117048).











