Protein-PEG Conjugation Technologies
The conjugation of PEG to peptides or proteins, known as PEGylation, has been widely used in therapeutic fields to improve the stability and biopharmaceutical performance. PEG is regarded as safe and there are many PEGylated protein drugs which have been approved by US Food and Drug Administration (FDA) in the market. BOC Sciences is a leading service provider of polymer bioconjugation. We have a good record in the development of polymer projects. We provide customized solutions from early detection of bioconjugation to development and clinical manufacturing.
Introduction of Protein-PEG Conjugation Technologies
Globular proteins can be denatured by external stress such as solvents, inorganic salts, exposure to acids orbases, and by heat, which alters their secondary and tertiary structures but retains the peptide bonds of the primary structure between the amino acids. Since all structural levels of the protein determine its function, the proteinis usually no longer bioactive once it has been denatured. However, unfolded proteins could be regarded as monodispersed biopolymers providing well-defined contour length and various functional groups at determined positions along the main chain. In 2003, Whitesides et al. pioneered an approach for preparing linear polymers with determined chain lengths and functional groups at defined locations along the chain by acylation of denatured proteins. Since then, denatured proteins have emerged as a unique polymeric platform for the construction of defined nano-architectures and nanomaterials for various applications. The PEG side chains could reduce protein binding and provide "stealth properties" by shielding the immunogenic recognition sites (epitopes). Therefore, polypeptide-PEG conjugates based on denatured proteins provide various attractive features for biomedical applications and as precision substrates for templated synthesis of well-defined nanomaterials.
 Fig. 1. Hyperbranched PEG supported photonic nanostrucutures for optical hydrogels (Chem. Mater. 2018, 30(17): 609-6098).
Fig. 1. Hyperbranched PEG supported photonic nanostrucutures for optical hydrogels (Chem. Mater. 2018, 30(17): 609-6098).
For protein denaturation, protein aggregation during the denaturation process needs to be strictly avoided as it is very challenging to disaggregate the protein agglomerates once they have precipitated, which reduces yields and makes purification more difficult. Typically, chaotropic agents such as urea to break hydrogen bonds and other supramolecular forces and mild reducing agents such as tris(2-carboxyethyl) phosphine(TCEP) are added. Stabilizing hydrophilic polymer chains can be attached to the polypeptide backbone before or after the denaturation step to prevent aggregation of the denatured polypeptide chains. In comparison to synthetic polymers, the most prominent advantages of denatured proteins are their monodisperse lengths and defined amino acid sequences. Therefore, the denatured protein-PEG conjugates can beused as precision templates for the preparation of various structurally defined nanomaterials.
Applications of Protein-PEG Conjugates
Nanoparticle Coatings
Denatured protein-PEG conjugates serve as attractive nanoparticle coatings due to the availabilities of many reactive amino-, carboxylic acid and thiol groups that could interact with various nanoparticle surfaces though electrostatic interactions or hydrogen bonds as well as the presence of hydrophobic amino acids that bind hydrophobic surfaces by van der Waals interactions.
Metal Nanoparticles
Due to the presence of abundant amino groups in the backbone, denatured protein-PEG conjugates possess strong capability to bind metal ions. Therefore, the biopolymer providing high water solubility was used as an ideal substrate for templated synthesis of metal nanoparticles.
Hybrid Hydrogels
In combination with various crosslinking chemistries, the denatured protein-PEG conjugate served as biocompatible and biodegradable high molecular weight scaffold to prepare injectable hybrid hydrogels. As crosslinkers, multi-arm DNA as well as self-assembling peptide sequences have been applied.
Core-shell Nanostructures
The architecture of denatured protein-PEG conjugates responds to changes of the balance of hydrophilicand groups along the polypeptide backbone. These changes could either be lipophilic functionalities that are covalently attached or the presence of hydrophobic guest moieties that interact with the lipophilic amino acid side chains via supramolccular interactions. In this way, well-defined core-shell nanostructures were formed suitable for catalysis and delivery of lipophilic molecules into cells.
Our Structural Design of Polymer Bioconjugates

Advantages of Protein-PEG Conjugates
- Biocompatibility
- Biodegradability of proteases
- Defined peptide sequence
- Tunable transition between globular, collapsed andextended architectures
- Various functionalities in specific positions which allow the realization of complextasks such as cellular uptake and intracellular delivery
- The final polymers offer narrow molecular weight distributions that can be characterized by mass spectrometry ensuring thequality control of products
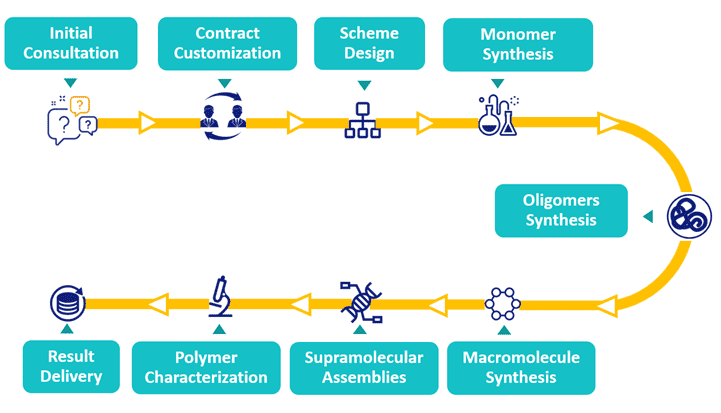
Our Polymer Bioconjugation Workflow
References
- Weil, T. et al. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. Progress in Polymer Science. 2020, 105: 101241.
- Whitesides, G.M. et al. Synthesis of monodisperse polymers from proteins. J. Am. Chem. Soc. 2003, 125: 12392-3.

 Fig. 1. Hyperbranched PEG supported photonic nanostrucutures for optical hydrogels (Chem. Mater. 2018, 30(17): 609-6098).
Fig. 1. Hyperbranched PEG supported photonic nanostrucutures for optical hydrogels (Chem. Mater. 2018, 30(17): 609-6098).



