- CAS: 1003-14-1
- Molecular Weight: 86.15
- Molecular Formula: C5H10 O
- CAS: 10276-21-8
- Molecular Weight: 154.21
- Molecular Formula: C9H14 O2
- CAS: 106-86-5
- Molecular Weight: 124.18
- Molecular Formula: C8H12O
- CAS: 114205-89-9
- Molecular Formula: C33H30O4
- CAS: 122413-85-8
- Molecular Weight: 228.31
- Molecular Formula: C12H22NO3
- CAS: 1239602-38-0
- Molecular Weight: 306.47
- Molecular Formula: C14H30O5Si
- CAS: 1436-34-6
- Molecular Weight: 100.16
- Molecular Formula: C6H12O
- CAS: 15965-99-8
- Molecular Weight: 298.5
- Molecular Formula: C19H38O2
- CAS: 1686-14-2
- Molecular Weight: 152.23
- Molecular Formula: C10H16O
- CAS: 1689-71-0
- Molecular Weight: 196.24
- Molecular Formula: C14H12O
- CAS: 18180-30-8
- Molecular Weight: 112.13
- Molecular Formula: C6H8O2
- CAS: 1838-94-4
- Molecular Weight: 84.12
- Molecular Formula: C5H8O
- CAS: 18401-43-9
- Molecular Weight: 256.41
- Molecular Formula: C13H24O3Si
- CAS: 18724-32-8
- Molecular Weight: 382.69
- Molecular Formula: C20H38O3Si2
- CAS: 1888-89-7
- Molecular Weight: 114.14
- Molecular Formula: C6H10O2
- CAS: 19780-16-6
- Molecular Weight: 296.53
- Molecular Formula: C20H40O
- CAS: 19932-26-4
- Molecular Weight: 188.12
- Molecular Formula: C6H8 F4 O2
- CAS: 19932-27-5
- Molecular Weight: 288.14
- Molecular Formula: C8H8F8O2
- CAS: 20217-01-0
- Molecular Weight: 307.97
- Molecular Formula: C9H8Br2O2
- CAS: 20780-54-5
- Molecular Weight: 120.15
- Molecular Formula: C8H8O
What Are Epoxide Monomers?
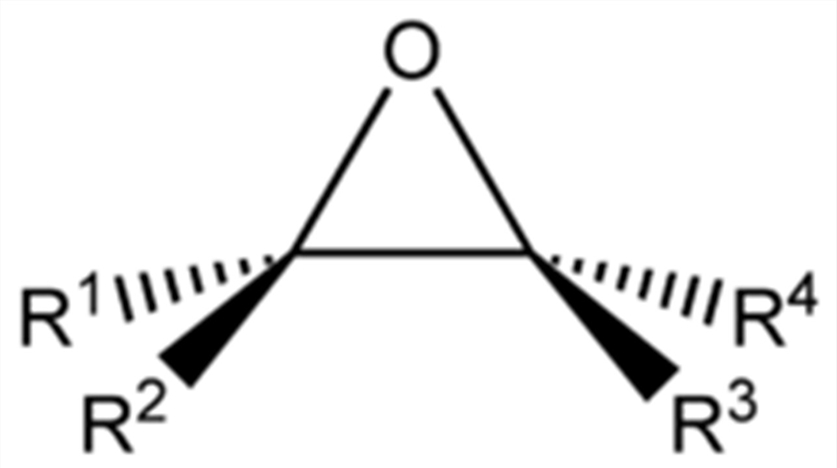
Epoxides contain three-membered ether structures in which three atoms are in a plane that approximates an equilateral triangular structure. They can be regarded as derivatives of ethylene oxide. As a class of important structural units, epoxide monomers are important synthons in organic synthesis and polymer synthesis. Common epoxides include ethylene oxide and propylene oxide, which are primarily used as biocides, diluents, and precursors for polyether polyols. Some epoxides such as styrene oxide are important pharmaceutical intermediates.
The oxygen and carbon atoms in the three-membered ring structure of epoxides have strong nucleophilic and electrophilic properties, respectively, which increase the ring tension and make them highly reactive. This determines that epoxide monomers are a class of reactive chemicals that can be used to carry out various reactions.
How Are Epoxide Monomers Synthesized?
At present, the production processes of producing epoxides from olefins can be mainly classified into four types: chlorohydrin process, hydroperoxide process, hydrogen peroxide composite process and direct epoxidation process.
Chlorohydrin process is the earliest technology to produce epoxides, and its selectivity can exceed 90%. However, due to the large amount of brine by-products that cause environmental pollution, it is gradually replaced by greener and more environmentally friendly processes. Hydroperoxide process first requires the oxidation of alkanes to produce alkyl hydroperoxides, which further oxidize olefins to produce epoxides. The entire process produces a fixed proportion of by-products. Hydrogen peroxide synthesis process is a green and efficient technology. Among them, hydrogen peroxide is synthesized in situ, which can further reduce the production cost. Direct epoxidation process refers to the use of metal-catalyzed olefins and molecular oxygen to directly obtain epoxides.
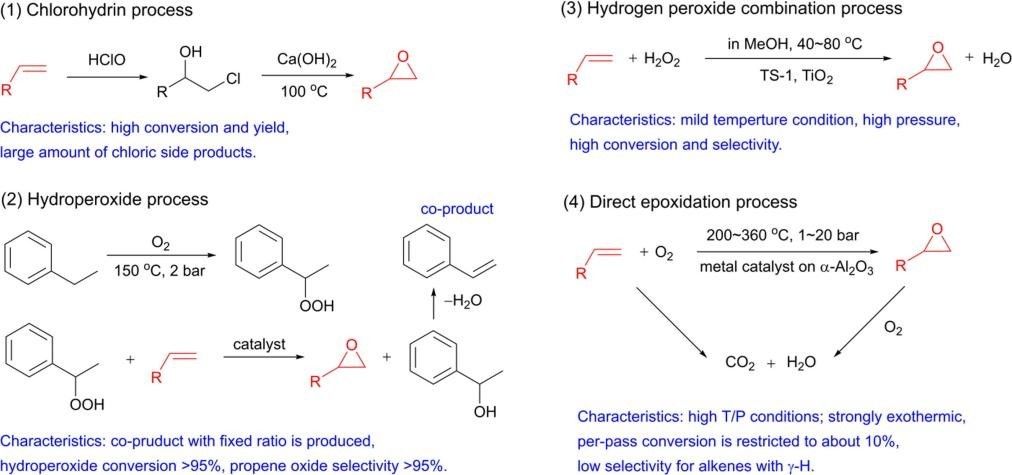 Fig. 1 Four representative processes to produce epoxides from alkenes.[1]
Fig. 1 Four representative processes to produce epoxides from alkenes.[1]
Reactions of Epoxide Monomers
Epoxides have been used in organic synthesis due to their ready availability in racemic and enantiomerically enriched/pure forms and their ability to undergo intermolecular and intramolecular ring-opening reactions with a wide variety of carbon and heteroatom nucleophiles.
- Epoxide-CO2 Copolymerization
Epoxides can be copolymerized with carbon dioxide in the presence of different coordination catalysts to form polycarbonates. The copolymerized CO2-based polycarbonate material has degradability. Therefore, this copolymerization effectively solves the two major challenges of green sustainable development: greenhouse effect and white pollution. The development of efficient catalysts is crucial for this polymerization reaction. In recent years, various catalysts have been developed including lanthanide metal trivalent complexes, double metal cyanide complexes, complexes formed by metal ions and multidentate ligands, and dimerized zinc complexes.
- Epoxide-Arene Cyclizations
Epoxide-arene cyclizations are a class of intramolecular reactions that represent a special type of epoxide derivatization. This reaction can be used to synthesize various benzo-fused carbocyclic and heterocyclic compounds in a chemoselective, regioselective and stereoselective fashions.
If you are interested in our epoxide monomer products, please contact us immediately!
References
- Zy A, et al. Microreaction processes for synthesis and utilization of epoxides: A review. Chemical Engineering Science. 2020, 229.
- Das S K. Recent Advances in the Intramolecular Reactions of Epoxides with Arenes and Heteroarenes. Asian Journal of Organic Chemistry. 2017, 6(3).



![7-Oxabicyclo[4.1.0]heptan-2-one,4,4,6-trimethyl-](https://resource.bocsci.com/structure/10276-21-8.gif)










![1,3 BIS[2(3,4 EPOXYCYCLOHEX-1-YL)ETHYL]TETRA-METHYLDISILOXANE](https://resource.bocsci.com/structure/18724-32-8.gif)


![Oxirane,2-[(2,2,3,3-tetrafluoropropoxy)methyl]-](https://resource.bocsci.com/structure/19932-26-4.gif)
![Oxirane,[[(2,2,3,3,4,4,5,5-octafluoropentyl)oxy]- methyl]-](https://resource.bocsci.com/structure/19932-27-5.gif)



 Fig. 1 Four representative processes to produce epoxides from alkenes.[1]
Fig. 1 Four representative processes to produce epoxides from alkenes.[1]











