Polymerization Initiators
Browse by
Introduction
Polymerization initiators are a chemical process of some chemical species which reacts with the monomer to make an intermediate compound which will link consecutively with an outsized number of the latest monomers into the polymeric compound. The initiation systems of free radical polymerization can be summarized as azo initiators, peroxides initiators, redox initiators, multifunctional initiators and macromolecular initiators.
Types of Polymerization Initiators
Azo initiators are the earliest initiators of free radical polymerization, which have no induced decomposition and produce only one kind of free radical. For example, V-50 initiator is applied in aqueous polymerization and emulsion polymerization of a variety of vinyl monomers word-widely due to its advantage of non-toxic decomposition products, high conversion rate, no residue and agglomeration in the polymerization process.
Peroxide initiators are a class of compounds containing peroxyl (-O-O-), which breaks and splits into two corresponding free radicals after being heated to initiate the polymerization of monomers. For example, benzoyl peroxide (BPO) is one of the most widely researched organic peroxide initiators, which has been successfully applied in the polymerization and co-polymerization of a variety of monomers ascribe to its mature synthesis process, moderate decomposition temperature and half-life.
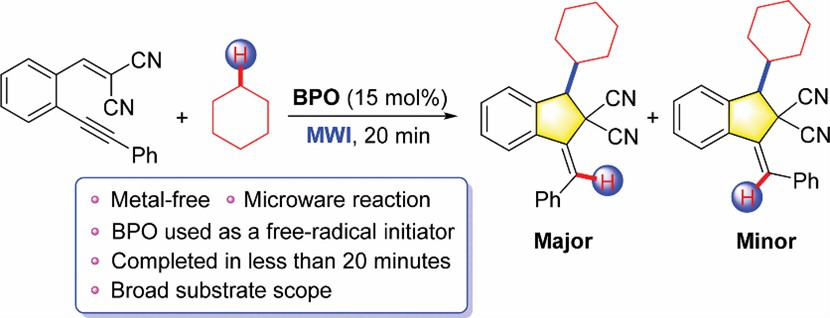 (Organic & Biomolecular Chemistry. 2022, 20(18): 3817-3822.)
(Organic & Biomolecular Chemistry. 2022, 20(18): 3817-3822.)
Compared with the polymerization of monomers initiated by peroxide, the use of redox initiators can reduce the decomposition activation energy, so that the polymerization can be carried out at a lower temperature, which is conducive to saving energy and improving the performance of the polymer. For example, amines as reducing agents are the most commonly used organic redox initiating systems.
- Multifunctional Initiators
Multifunctional initiators are initiators which have two or more active groups to produce free radicals in their molecular structure. The advantage is that the decomposition order and rate of the active group in the initiator can be controlled by optimizing the reaction temperature to obtain a higher polymer relative molecular weight and polymerization rate. Moreover, the copolymer with specific properties can be designed by changing the order of monomer addition. For example, tetro butyl peroxyl tetracarbonate (JWEB50) is a typical oxide initiator, which was developed to applied for the free radical polymerization of styrene.
- Macromolecular Initiators
Macromolecular initiators refer to a high molecular compound containing a radical group in the molecular chain. The polymer with active end is synthesized by active polymerization, which is then used as macromolecular initiator to initiate the polymerization of other monomers, and the block or graft copolymers with preset structure are composed.
If you are interested in our polymerization initiators, please contact us immediately!
References
- Zhou, Y.N. et al. Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering. Progress in Polymer Science. 2022, 130: 101555.
- Yang, Y. et al. A facile, green, and tunable method to functionalize carbon nanotubes with water-soluble azo initiators by one-step free radical addition. Applied Surface Science. 2010, 256(10): 3286-3292.
- Ruan, S. et al. Microwave-accelerated and benzoyl peroxide (BPO)-initiated cyclization of 1,5-enynes having cyano groups with cyclic alkanes under metal-free conditions. Organic & Biomolecular Chemistry. 2022, 20(18): 3817-3822.
- Garra, P. et al. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Progress in Polymer Science. 2019, 94: 33-56.


 ATRP Initiators
ATRP Initiators

 (Organic & Biomolecular Chemistry. 2022, 20(18): 3817-3822.)
(Organic & Biomolecular Chemistry. 2022, 20(18): 3817-3822.)












