- CAS: 101-43-9
- Molecular Weight: 168.23
- Molecular Formula: C10H16O2
- CAS: 10475-46-4
- Molecular Weight: 212.24
- Molecular Formula: C14H12O2
- CAS: 10595-06-9
- Molecular Weight: 206.23776
- Molecular Formula: C12H14O3
- CAS: 10595-80-9
- Molecular Weight: 194.21
- Molecular Formula: C6H10O5S
- CAS: 106-71-8
- Molecular Weight: 125.12
- Molecular Formula: C6H7NO2
- CAS: 111918-90-2
- Molecular Weight: 202.32
- Molecular Formula: C9H18O3Si
- CAS: 1126272-77-2
- Molecular Weight: 259.77
- Molecular Formula: C13H22ClNO2
- CAS: 114859-23-3
- Molecular Weight: 266.16
- Molecular Formula: C11H7F5O2
- CAS: 121177-93-3
- Molecular Weight: 204.3
- Molecular Formula: C8H16O4Si
- CAS: 121601-93-2
- Molecular Weight: 206.28
- Molecular Formula: C13H18O2
- CAS: 130224-95-2
- Molecular Weight: 170.16
- Molecular Formula: C8H10O4
- CAS: 1314713-40-0
- Molecular Weight: 377.45
- Molecular Formula: C16H27NO7S
- CAS: 13159-52-9
- Molecular Weight: 178.61
- Molecular Formula: C7H11ClO3
- CAS: 1330-61-6
- Molecular Weight: 212.33
- Molecular Formula: C13H24O2
- CAS: 13402-02-3
- Molecular Weight: 296.49
- Molecular Formula: C19H36O2
- CAS: 13532-94-0
- Molecular Weight: 186.2481
- Molecular Formula: H2C=C(CH3)CO2CH2CH2O(CH2)3CH3
- CAS: 13641-96-8
- Molecular Weight: 141.12
- Molecular Formula: C6H7NO3
- CAS: 13642-97-2
- Molecular Weight: 252.14
- Molecular Formula: C10H5F5O2
- CAS: 13688-55-6
- Molecular Weight: 144.24
- Molecular Formula: C6H12O2Si
- CAS: 13688-56-7
- Molecular Weight: 158.27
- Molecular Formula: C7H14O2Si
Introduction
The monofunctional acrylate determines the type and nature of the backbone polymer backbone. The monomers are chosen to obtain the desired glass transition temperature, flexibility, mechanical strength, polarity and hydrophilic/hydrophobic properties of the resulting polymer. In general, acrylates polymerize very readily in the presence of light and heat. These polymers are known for their transparency, fracture resistance and elasticity. Acrylate polymers are commonly used as binders in cosmetics such as nail polish. Currently, acrylate monomers are mostly classified into Acrylate-Difunctional, Acrylate-Monofunctional and Acrylate-Multifunctional.
Due to the increasing variety of acrylate monomers, the structures of acrylate dispersants are also diverse. Dimethylethanolamine acrylate is a common anchoring group monomer in acrylate hyperdispersants, and has good adsorption capacity for organic pigments such as carbon black. Generally, the amino anchoring group inside the dispersant is mainly a tertiary amine or quaternary ammonium salt, mainly to prevent the reaction with the curing agent in the PU system, and the dispersant containing primary amine is rarely used in the PU system. In addition to the amino group, there is also an anchoring group that is an aromatic ring (such as a benzene ring, a naphthalene ring, etc.). This anchoring group is mainly anchored with pigment particles through the conjugation of P electron cloud, many common color paste dispersants contain this group.
Applications
Presently, the main application of monofunctional acrylate is the synthesis of polyacrylate compound, which is a common polymer material with good light resistance and gloss. Acrylate polymers are readily soluble in acetone, ethyl acetate, benzene, and dichloroethane, but insoluble in water. Due to the flexibility of their polymer chains, the glass transition temperature (Tg) of acrylate polymers is low and varies with the number of carbon atoms in the ester group and its branches. In addition, acrylate polymers have adhesive properties and can be used as pressure-sensitive and heat-sensitive adhesives. Additionally, acrylates are the most commonly used polyurethane modifiers. In the field of polyurethane, the use of acrylate polymers is as follows:
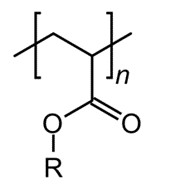
- Synthesis of polyacrylate polyols, used as raw materials for acrylic-polyurethane coatings for automotive coatings, exterior coatings, etc.
- Synthetic polyurethane-acrylate emulsions for leather finishers, fabric coatings, coatings.
- Acrylic paints.
- Acrylic fibre.
- Acrylic resin as pressure-sensitive adhesive.
- Acrylic glass, also known as plexiglass.
- Reaction of hydroxyalkyl acrylate such as hydroxyethyl acrylate with polyurethane prepolymer for the preparation of UV-curable PUA coatings.
Effect of Acrylate Composition on Polymers
- Effect of Groups on Side Chains
When the side chain group of acrylate increases, the mobility of the side chain segment increases accordingly, which leads to an increase in specific heat capacity, which in turn reduces tensile strength and increases elongation.
- Composition of Polymer Main Chain
The relationship between macroscopic film properties of acrylates and polymer composition can be described in terms of glass transition temperature and solubility parameters.
If you are interested in our acrylate-monofunctional, please contact us immediately!













ammonio]propane-1-sulfonate](/images/logo.svg)






















