- CAS: 10443-65-9
- Molecular Weight: 150.96
- Molecular Formula: C3H3BrO2
- CAS: 3586-58-1
- Molecular Weight: 100.116
- Molecular Formula: C5H8O2
- CAS: 381-98-6
- Molecular Weight: 140.06
- Molecular Formula: C4H3F3O2
- CAS: 38862-25-8
- Molecular Weight: 183.16
- Molecular Formula: C8H9NO4
- CAS: 5650-75-9
- Molecular Weight: 114.14
- Molecular Formula: CH3(CH2)2(=CH2)CO2H
- CAS: 72707-66-5
- Molecular Weight: 164.99
- Molecular Formula: C4H5BrO2
Introduction

Acrylic acid, also known as prop-2-enoic acid, is an organic molecule with the formula C3H4O2 and is one of the simplest unsaturated carboxylic acids. At room temperature, acrylic acid is a colorless liquid with a tart or acrid smell.[1] In recent years, the demand for acrylic acid has been increasing year by year, and the growth rate is expected to exceed 4% in the next few years. The main processes for producing acrylic acid include chlorohydrin method, low-pressure oxo synthesis method, high-pressure oxo synthesis method, formaldehyde acetic acid method, hydrolysis of acrylonitrile method, ethylene method, propylene direct oxidation method, etc.[2] However, the chlorohydrin method, low-pressure oxo synthesis method, and high-pressure oxo synthesis method are gradually being replaced due to high raw material costs, large consumption, and low efficiency. The ethylene process has gradually developed in recent years, but due to the imperfection of the process, the process technology is still in its infancy, and there is no large-scale ethylene process yet.
Currently, most acrylic acid is produced industrially by the direct oxidation of propylene. As shown in Fig 1, the first step is the oxidation of propylene to produce acrolein, that is, propylene, water vapor and air (almost only oxygen in the air) pass through the catalyst layer of the reactor in a certain proportion. Under the action of the oxidation catalyst, the gas is oxidized to produce a large amount of acrolein and negligible amount of acrylic acid. Then, the oxidation of acrolein to acrylic acid is the second stage. Through the reactor, acrolein is converted to acrylic acid under the action of Mo-V catalyst.
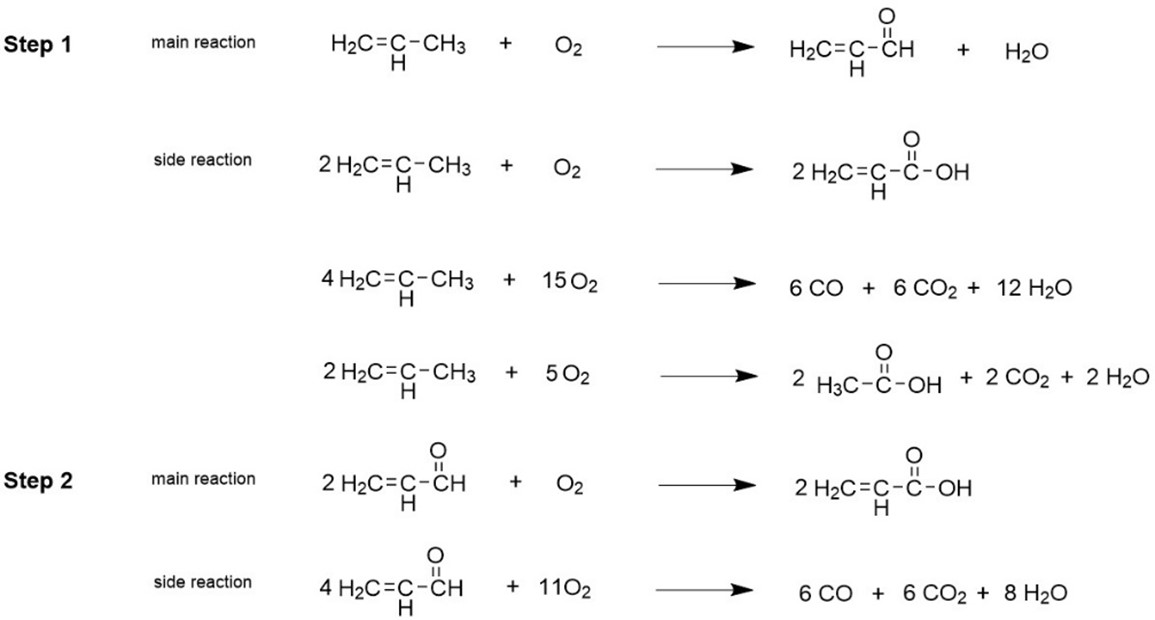 Fig. 1. The direct oxidation of propylene synthesis process of acrylic acid.
Fig. 1. The direct oxidation of propylene synthesis process of acrylic acid.
Application
- Acrylate Synthesis. Acrylic acid is a very important organic chemical raw material and an important derivative of propylene. It is commonly used in the manufacture of acrylates, such as methyl acrylate, ethyl acrylate, butyl acrylate, etc. These monomers can react with hard monomers such as styrene and vinyl acetate to produce a variety of acrylic resin products, which can be widely used in coatings, chemical fibers, textiles, leather, adhesives, acrylic rubber and other fields.
- Polyacrylic Acid Synthesis. Polyacrylic acid ([C3H4O2]n), the polymerization product of acrylic acid monomers, is also known as acrylic resin. Polyacrylic acid has a wide range of application in many industrial fields, including as a lithium battery binder, scale inhibitor, quenching agent, and pigment dispersant.[3]
If you are interested in our acrylic acids, please contact us immediately!
References
- Brown, D. Acrylic Acid. Encyclopedia of Toxicology (Third Edition). 2014, Pages 74-75.
- Sun, Y.N. et al. Application and Production Technology of Acrylic Acid. Petrochemical Industry Technology. 2022, 29(08): 258-260.
- Yang, J.S. et al. Suppressing Voltage Fading of Li-Rich Oxide Cathode via Building a Well-Protected and Partially-Protonated Surface by Polyacrylic Acid Binder for Cycle-Stable Li-Ion Batteries. Adv Energy Mater. 2020, 10(15): 201904264.









 Fig. 1. The direct oxidation of propylene synthesis process of acrylic acid.
Fig. 1. The direct oxidation of propylene synthesis process of acrylic acid.











