Lactide and Glycolide Polymers
- Molecular Formula: C6H11O3((((C3H4O2)x(C2H2O2)y)m)H)3
- Molecular Formula: C5H8O4((((C3H4O2)x(C2H2O2)y)m)H)4
- Molecular Formula: (C3H4O2)x(C2H2O2)y-1C3H6O2
- Molecular Formula: CH3(C3H4O2)m(C4H6O3)nCH3
- Molecular Formula: (C4H6O3)m(C4H4O4)n
- Molecular Formula: (C4H6O3)m(C6H8O4)n
- Molecular Formula: [C3H4O2]x[C2H2O2]y
- Molecular Formula: [C3H4O2]x[C2H2O2]y
- Molecular Formula: H(C3H4O2)nOCH3
- Molecular Formula: C6H10O3(C6H8O4)n
- Molecular Formula: (C3H4O2)nC5H8O3
- Molecular Formula: C3H9NO(C3H4O2)n
- Molecular Formula: H(C3H4O2)nC3H6ON3
- Molecular Formula: H(C3H4O2)nC3H3O
- Molecular Formula: H(C3H4O2)nOC2H4SH
- Molecular Formula: (C6H8O4)m(C6H8O4)n
- Molecular Formula: (C4H4O4)m(C6H8O4)n
- Molecular Formula: CH3[C6H8O4]m[C4H6O3]nCH3
- Molecular Formula: CH3(C4H6O3)m(C4H4O4)n(C6H8O4)oCH3
- Molecular Formula: CH3(C4H6O3)m(C6H8O4)nCH3
Introduction
Lactide and ethyl glycolide polymers are polymer materials synthesized from lactic acid (LA) and glycolic acid (GA). Typically, lactide is produced from lactic acid by a process involving the steps of polylactic acid forming lactic acid oligomers, which forms lactide in the presence of catalysts. Lactide is a colorless transparent flake or needle crystal with a melting point of 93-95°C, easily soluble in chloroform and ethanol, but insoluble in water. Glycolide is a white flaky crystal, and the unclean ethyl ester is light yellow, with a melting point of 84°C and a boiling point of 330°C, soluble in ethyl acetate. The chemical structure of glycolide is shown in Fig 1.[1] Lactide and ethyl glycolide polymers have many excellent properties, including non-toxicity, non-irritation, good in vivo biocompatibility and absorbability. Lactide and ethyl glycolide polymers can be metabolized into LA and GA in the human body, and further degraded into water and carbon dioxide by enzymatic catalysis. With excellent biocompatibility, they have been officially approved as medicinal excipients by FDA in 1997.[2]
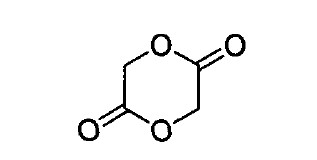 Fig. 1. Chemical structure of glycolide.
Fig. 1. Chemical structure of glycolide.
Characteristics
The degradation rate of lactide and glycolide polymers can be controlled to a certain extent by adjusting the molecular weight of copolymer and the content ratio of LA and GA in the copolymer. Therefore, they have excellent properties such as good biocompatibility and adjustable degradation rate. Specific characteristics include the following aspects:
- Good biocompatibility
- Stability
- Easy polymerization
- Transparency
Application
Based on their excellent properties, lactide and glycolide polymers are widely used in medical industry and plastic packaging industry.
In the pharmaceutical industry, lactide and glycolide polymers are mainly used in the preparation of polyester drug-loaded microspheres, subcutaneous implants, sustained-release gels, and degradable surgical consumables. After biodegradation, the metabolites of lactide and glycolide polymers are non-toxic and can be excreted through the excretory system such as the kidneys. Lactide and Glycolide polymers are safe and stable to the human body and have slow-release and long-lasting effects. In addition, glycolide, as a material for surgical sutures, can overcome the traditional shortcomings of unstable intestinal absorption, uneven composition, easy rejection, and easy inflammation.
Lactide and glycolide polymers have high hardness and high light transmittance, and can be modified by blending with other materials, so they are widely used to prepare degradable plastics. Its performance can meet the requirements of use and remain stable during the storage period, and can be degraded into environmentally harmless substances under natural environmental conditions after use.
If you are interested in our lactide and glycolide polymers, please contact us immediately!
References
- Gupta, P.K. et al. Recent trends in biodegradable polyester nanomaterials for cancer therapy. Materials Science and Engineering C-Materials for Biological Applications. 2021, 127.
- Unal, S. et al. Poly(caprolactone) containing highly branched segmented poly(ester urethane)s via A(2) with oligomeric B-3 polymerization. Journal of Polymer Science Part A-Polymer Chemistry. 2008, 46 (18): 6285-6295.


.gif)
.gif)
-cooh.gif)
.gif)
.gif)
.gif)
-fluorescein.gif)
-rhodamine b.gif)
.gif)
 2-hydroxyethyl, methacrylate terminated.gif)
, acrylate terminated.gif)
, amine terminated.gif)
, azide terminated.gif)
, propargyl terminated.gif)
, thiol terminated.gif)

.gif)
.gif)

.gif)

 Fig. 1. Chemical structure of glycolide.
Fig. 1. Chemical structure of glycolide.












