Chitosan Film/Membrane Preparation
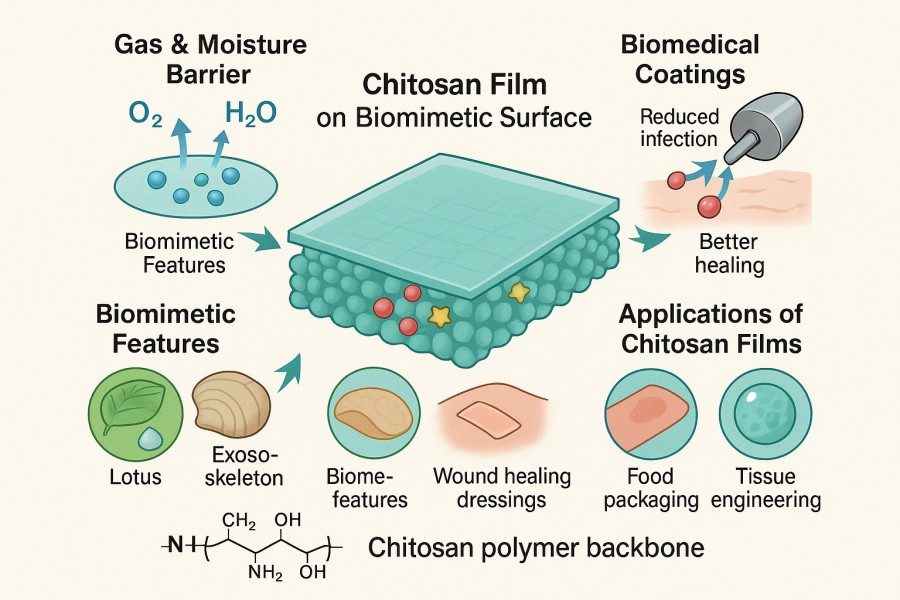
Chitosan films and membranes are sheet-like materials primarily made from chitosan, prepared through solution casting, coating, freeze-drying, or other methods, and possess defined thickness and mechanical strength. Films generally refer to thin, transparent, and flexible sheets suitable for packaging, coating, and miniaturized applications. Membranes focus on pore structure and selective permeability, commonly used for filtration, separation, and functional carriers. Chitosan-based materials combine biocompatibility and processability, and can be modified physically or chemically to achieve various functionalities, including antibacterial activity, drug loading, and cell support. BOC Sciences specializes in high-quality chitosan film preparation and development services, providing comprehensive support for researchers, material developers, and manufacturers. Our services cover the entire process from raw material selection, film preparation, and functional modification to final application validation, helping clients efficiently develop chitosan films and membrane materials tailored to their research or production needs.
What We Offer
High-Quality Chitosan Film & Membrane Fabrication Services
We offer a wide range of chitosan film materials, customizable for different applications, including thickness, pore structure, mechanical properties, and specialized functions (e.g., antibacterial, conductive, or stimuli-responsive). Whether the project involves developing high-strength tissue engineering scaffolds, smart drug delivery systems, or flexible biomimetic electronic films, BOC Sciences provides professional material solutions and technical guidance to ensure the films meet performance, functionality, and process requirements.
Pure Chitosan Films
- Prepared from high-purity chitosan, offering excellent biocompatibility and biodegradability.
- Suitable for basic film research, simple wound dressings, or as foundational drug carrier films.
- Thickness, transparency, and mechanical properties can be adjusted according to requirements.
Crosslinked Chitosan Films
- Enhanced intermolecular interactions through chemical or physical crosslinkers to improve mechanical strength and stability.
- Degradation rate and pore structure can be tailored for tissue engineering scaffolds and controlled-release drug films.
- Supports multiple crosslinking methods, including chemical crosslinking (glutaraldehyde, polyacids) or ionic crosslinking (Ca²⁺, Zn²⁺).
Multilayer/Composite Chitosan Films
- Combining chitosan with other natural or synthetic polymers (e.g., collagen, gelatin, hyaluronic acid) or nanoparticles to form multilayered or composite structures.
- Customizable functions such as antibacterial, conductive, stimuli-responsive, or drug carrier capabilities.
- Widely applied in tissue engineering, smart drug delivery systems, and flexible biomimetic films.
Porous/Nanoporous Chitosan Films
- Prepared via freeze-drying, templating, or phase separation techniques, with precise control over pore size and porosity.
- Suitable for three-dimensional cell scaffolds, drug sustained release, multi-stage release, and molecular sieve applications.
Functionalized Chitosan Films
- Achieved through chemical modification or nano-composites to provide antibacterial, conductive, pH/temperature-responsive, or drug carrier functionalities.
- Enables customized solutions for smart biomimetic films, electronic skin, pressure sensors, and environment-responsive materials.
Nanocomposite Chitosan Films
- Nanomaterials such as nano-hydroxyapatite, carbon nanotubes, graphene, or nanocellulose are uniformly dispersed within chitosan films.
- Significantly enhances mechanical properties, conductivity, and bioactivity, meeting the needs of advanced tissue engineering scaffolds, conductive biomimetic films, and smart sensors.
Looking for Biomimetic Material Solutions?
From natural polymers to bio-inspired composites, BOC Sciences provides customized materials to accelerate your research and industrial applications.
Services
Personalized Chitosan Film Custom Development Services
BOC Sciences' preparation and customization services cover the full process from raw material selection, solution preparation, film casting, drying and crosslinking, to functional modification and performance optimization. Whether a transparent, flexible single-layer film or a porous, multifunctional composite film is needed, we provide tailored solutions. Additionally, we can adjust thickness, porosity, crosslinking methods, and functional modifications according to the client's application scenario, creating designs for tissue engineering scaffolds, drug delivery systems, smart sensor films, or eco-friendly separation membranes.
1Personalized Customization Services
BOC Sciences offers end-to-end customization from basic film preparation to functional film development. Services include:
- Formulation Customization: Optimize chitosan type, composite ratios, and solution preparation according to project needs.
- Process Customization: Select the most suitable film preparation method and optimize drying, crosslinking, and pore formation conditions.
- Functional Development: Achieve antibacterial, drug carrier, conductive, or stimuli-responsive functionalities as required.
- Scale-up Support: Provide lab-scale preparation, small-batch development, and pre-industrial production process optimization.
2Raw Material and Solution Preparation
BOC Sciences supplies high-purity chitosan and derivatives, with custom molecular weights, degrees of deacetylation, and solubility profiles. Services include:
- Chitosan Solution Preparation: Professional solutions for acid dissolution, pH adjustment, and additive incorporation, tailored to desired film thickness, mechanical performance, and porosity.
- Composite Film Precursors: Uniform mixing of chitosan with collagen, gelatin, hyaluronic acid, or nanoparticles to form composite film precursors.
- Custom Functional Solutions: Support preparation of antibacterial, conductive, stimuli-responsive, or drug carrier precursors.
3Film Casting and Drying
The casting and drying process directly affects film mechanical properties, transparency, and pore structure. BOC Sciences provides multiple options:
- Solution Casting: Evenly coat chitosan solution on molds, then dry to obtain smooth films, suitable for drug-controlled release or transparent film applications.
- Spin Coating: Ideal for ultrathin or miniaturized films, achieving nanoscale thickness control.
- Freeze-Drying: Used for porous films or 3D scaffolds, with precise pore control via temperature and freezing rate.
- Thermal Treatment and Crosslinking: Enhance mechanical strength and thermal stability through physical or chemical crosslinking, suitable for tissue engineering or industrial production.
4Film Performance Optimization and Functionalization
Application performance can be achieved through structural tuning and functional modification. BOC Sciences offers comprehensive optimization services:
- Mechanical Performance: High toughness, strength, or flexibility through crosslinkers, composite design, and thickness adjustment.
- Porosity and Permeability Control: Porous films prepared via freeze-drying, phase separation, or templating meet drug release, molecular sieve, or cell scaffold needs.
- Chemical Functionalization: Introduce antibacterial, pH-responsive, or conductive groups to meet specific functional requirements.
- Nanocomposite Development: Integrate nanoparticles, carbon nanotubes, graphene, or nano-hydroxyapatite with chitosan films to enhance mechanics, conductivity, and bioactivity.
Characterization
Analysis and Characterization of Chitosan Films
Accurate characterization of physical, chemical, and functional properties is critical for ensuring quality, performance, and reproducibility during chitosan film preparation and functional development. BOC Sciences provides comprehensive analytical and characterization services, covering structure, morphology, mechanical performance, pore features, and functional activity. Our systematic analytical approach helps clients gain in-depth insights into both microstructure and macroscopic properties, offering reliable data to support film R&D, functional modification, and industrial applications.
| Category | Analysis Item | Technique | Service Description |
|---|
| Physical Properties | Film Thickness | Digital Micrometer, Optical Microscope | Accurately measures the thickness range and uniformity of films, ensuring controllable preparation processes. |
| Tensile Strength | Universal Testing Machine | Determines the tensile strength, breaking stress, and elongation of films, providing data for mechanical optimization. |
| Flexibility & Bending | Bending Test, Flexibility Tester | Evaluates the stability and flexibility of films under various bending conditions, suitable for wearable and flexible applications. |
| Chemical Structure | Functional Group Identification | Fourier Transform Infrared Spectroscopy (FTIR) | Analyzes amino, hydroxyl, and other functional groups in the film, verifying chemical modification or crosslinking. |
| Molecular Structure Analysis | Nuclear Magnetic Resonance (NMR) | Provides chemical structure information of chitosan chains, confirming degree of deacetylation and composite structure. |
| Crystalline Structure | X-ray Diffraction (XRD) | Analyzes crystallinity and amorphous content of films, assessing stability and processability. |
| Microscopic Morphology | Surface Morphology | Scanning Electron Microscopy (SEM) | Examines surface smoothness, pore distribution, and composite uniformity to support structural optimization. |
| Porous Structure | Nitrogen Adsorption (BET), Pore Size Analysis | Accurately measures pore size, porosity, and specific surface area, supporting controlled release and separation membrane design. |
| Functional Performance | Antibacterial Activity | Inhibition Zone Test, Colony Counting | Measures the film's antibacterial efficacy, evaluating results of antibacterial functionalization. |
| Drug Release Profile | In vitro Release Testing | Assesses controlled release behavior of drug-loaded films, providing data for drug delivery system development. |
| Responsiveness | pH/Temperature/Ion Response Tests | Tests the film's response under different environmental conditions, supporting smart biomimetic membrane development. |
| Conductivity | Four-Point Probe, Electrochemical Testing | Measures film conductivity and electrochemical stability, suitable for electronic skin or sensor applications. |
Advantages
Benefits of Chitosan Film & Membrane Development

- Comprehensive Technical Capability: We provide end-to-end services from raw material selection, solution preparation, film fabrication, drying, and crosslinking to functional modification and performance optimization. Our services cover pure films, composite films, porous films, and nanocomposite films, ensuring high controllability in both structure and functionality.
- Highly Customizable Services: We can design film thickness, pore structure, mechanical properties, and functional characteristics—including antibacterial, conductive, stimuli-responsive, or drug carrier functionalities—tailored to the client's needs, enabling customized biomimetic film materials.
- Advanced Fabrication Techniques: Utilizing solution casting, spin coating, freeze-drying, and crosslinking optimization, we ensure uniform, stable, and controllable films. These techniques are suitable for both laboratory-scale trials and industrial production, maintaining batch-to-batch consistency.
- Multidimensional Performance Analysis: We offer physical, chemical, microstructural, and functional characterizations, including thickness, mechanical strength, pore structure, functional groups, antibacterial activity, drug release, and conductivity, supporting R&D optimization and functional validation.
- Application-Oriented Solutions: Closely aligned with applications such as tissue engineering scaffolds, drug-controlled release systems, smart biomimetic films, flexible sensors, and separation membranes, we provide customized film materials and technical consulting to accelerate R&D and product implementation.
- End-to-End Support from R&D to Mass Production: Supporting laboratory preparation, small-batch development, and industrial-scale production, we ensure stable and controllable film performance, enabling smooth transitions from R&D validation to large-scale manufacturing.
- Professional Team Assurance: Our experienced team of biochemistry and polymer materials experts provides technical support, experimental design, and performance optimization solutions to ensure projects progress efficiently and achieve optimal outcomes.
Service Process
Full-Process Custom Chitosan Film Fabrication Services
BOC Sciences offers a one-stop, fully customized preparation service from project conception to industrial-scale production, covering material selection, film fabrication, functional modification, and performance validation. Through rigorous process management and multidimensional technical support, we deliver high-quality, functionally controllable chitosan films, supporting tissue engineering, smart sensor films, drug delivery systems, and other biomimetic material projects.
1Requirement Analysis
Our service process begins with a thorough understanding of the client's project. We communicate closely with clients to clarify application scenarios, performance requirements, and special functional needs, including mechanical strength, pore structure, and functionalization. By fully understanding project objectives and R&D stages, we lay a solid foundation for subsequent film design and customized development, ensuring alignment with client requirements.
2Material Selection
Based on project goals and film performance requirements, BOC Sciences provides professional recommendations for chitosan raw materials and composites. We consider molecular weight, degree of deacetylation, and purity of chitosan, while evaluating the potential of other natural or synthetic polymers and nanomaterials for composites. Optimized material combinations provide the best basis for functionalization, mechanical performance, and biocompatibility, ensuring excellent film performance in applications.

3Film Fabrication and Process Optimization
We select the most suitable fabrication method for each project, including solution casting, spin coating, and freeze-drying, and optimize process parameters. Film thickness, pore structure, and uniformity are precisely controlled, ensuring structural stability and reproducibility. We also conduct process validation at laboratory and pilot scales to provide feasibility data and references for scale-up production.
4Functional Modification
BOC Sciences can implement various functionalizations on films, including antibacterial, conductive, stimuli-responsive (pH, temperature, or ion-sensitive), and drug carrier functionalities. Through chemical modification or nanocomposite technology, we impart specific bioactivity and intelligent responsiveness to films, meeting the high-end application needs of tissue engineering, smart sensors, and drug delivery systems.

5Performance Characterization and Validation
To ensure films meet expected performance, we provide comprehensive analysis and validation services, including mechanical properties, pore structure, surface morphology, chemical functional groups, functional activity, and drug release behavior. Multidimensional data allow clients to fully understand material properties and provide a scientific basis for process optimization and functional development.
6Customized Scale-Up Solutions
For small-scale and industrial production, we provide complete scale-up solutions and technical guidance, including process parameter optimization, batch consistency control, and functional verification. End-to-end support from R&D to mass production ensures efficient translation of chitosan film projects into practical applications.
Applications
Applications of Chitosan Films in Biomimetic Materials
Due to their natural biocompatibility, biodegradability, and functionalization potential, chitosan films have become core materials in biomimetic material research and development. Their applications cover tissue engineering, drug delivery, smart sensing, and environmentally responsive separation, offering diverse solutions for research and industrial development.
Tissue Engineering and Regenerative Medicine
Chitosan films/membranes can mimic the extracellular matrix (ECM), supporting cell adhesion, proliferation, and differentiation. Specific applications include:
- Scaffolds: Porous chitosan membranes form 3D scaffolds supporting bone, cartilage, or skin cell growth. Pore size is controlled via freeze-drying to allow tissue-like structures within the membrane.
- Wound Dressings: Films can directly cover wounds, maintaining a moist environment, preventing infection, and gradually degrading to support healing.
- Artificial Skin: Composite transparent chitosan films with collagen or hyaluronic acid create biomimetic skin materials with breathability and cell compatibility.
Chitosan Film in Drug Delivery Systems
The porous structure and functional groups of chitosan membranes make them excellent drug carriers for controlled or targeted release. Applications include:
- Transdermal Patches: Chitosan films can load drugs for slow release through the skin.
- Microsphere-Loaded Films: Drugs encapsulated in microspheres are incorporated into chitosan films for multi-stage release and enhanced stability.
- Oral Dissolving Films: Rapidly dissolving films for localized oral drug delivery provide a convenient administration route.
Biomimetic Skin and Sensors
The flexibility and modifiability of chitosan films make them ideal for electronic skin and sensor applications:
- Pressure and Humidity Sensors: Incorporating conductive nanomaterials such as carbon nanotubes or graphene enables flexible pressure sensors for tactile detection in biomimetic skin.
- Smart Responsive Films: Chemically modified chitosan films respond to pH, temperature, or specific ions for environmental monitoring or biosignal detection.
Biomimetic Membranes and Separation Materials
In biomimetic membrane technology, chitosan films mimic biological membrane structures for water treatment, gas separation, and nutrient sieving:
- Ion-Selective Membranes: Amino groups in chitosan membranes selectively bind specific ions for targeted separation.
- Nanocomposite Membranes: Chitosan combined with inorganic nanomaterials enhances mechanical strength and selective permeability for biomimetic filtration systems.
FAQs
Frequently Asked Questions
How to make chitosan film?
The preparation of chitosan films typically begins by dissolving chitosan raw material in an acidic solution to form a homogeneous solution. This solution can then be converted into continuous films via casting, spin coating, or freeze-drying. During preparation, film thickness, pore structure, and drying conditions can be controlled to achieve tunable material properties. Functional modification or composite processing can also be applied to meet the requirements of biomimetic material development, such as tissue engineering, drug-controlled release, and smart film applications.
What is the characterization of chitosan films?
Characterization of chitosan films includes mechanical properties, thickness, pore structure, surface morphology, chemical functional groups, and functional attributes such as antibacterial activity and drug release capability. Systematic analysis of the film's mechanical, structural, and functional parameters allows assessment of stability, uniformity, and reproducibility. These characterization data provide a scientific basis for process optimization and functional development, helping researchers and developers select appropriate films for efficient application in tissue engineering, smart materials, and drug delivery.
What is a chitosan membrane?
A chitosan membrane is a film material based on the natural polysaccharide chitosan, prepared through solution casting or composite methods. It exhibits excellent biocompatibility, biodegradability, and functionalization potential. Chitosan membranes can serve as tissue engineering scaffolds or drug delivery carriers, and are also applicable in smart biomimetic films, flexible sensors, and environmentally responsive separation membranes. Through different fabrication and functionalization approaches, chitosan membranes meet diverse biomimetic material development needs, providing reliable, high-performance solutions for both research and industrial applications.
What is the chitosan membrane structure?
The structure of chitosan membranes consists of polymer chains formed via crosslinking or composite integration, resulting in uniform thickness, microscopically smooth surfaces, and tunable porosity. Membrane structure can be optimized using chemical crosslinkers, nanofillers, or multilayer composites to adjust mechanical performance, porosity, and surface characteristics. Precise structural design not only ensures mechanical stability but also provides a foundation for functional modification, allowing chitosan membranes to exhibit excellent performance in tissue engineering scaffolds, drug delivery, and smart biomimetic films.
What are chitosan membrane uses?
Chitosan membranes have broad applications in the biomimetic material field, including tissue engineering scaffolds, drug-controlled release systems, smart biomimetic films, flexible sensors, and environmentally responsive separation membranes. Their natural biocompatibility, biodegradability, and functionalization potential support cell growth, drug delivery, and environmental responsiveness. Membranes can be modified to enhance antibacterial, conductive, or stimuli-responsive properties, making them an essential choice for developing high-performance biomimetic films, smart materials, and environmental functional membranes in both research and industrial applications.
How is chitosan membrane preparation performed?
Chitosan membrane preparation involves dissolving chitosan, homogenization, film casting or spin coating, and drying, with optional chemical or physical crosslinking for functional modification. During preparation, film thickness, porosity, and mechanical properties can be adjusted to meet different R&D stages and industrial production requirements. Through process optimization, membranes maintain stable performance and functionality in laboratory, pilot, and mass production, enabling smooth translation from fundamental research to industrial applications.
How does chitosan film formation occur?
Chitosan film formation relies on solution casting, spin coating, or freeze-drying techniques. After uniformly spreading the solution, drying or solidification forms a continuous film layer, with thickness, pore structure, and uniformity adjustable according to application requirements. Functional modification or composite material addition can be performed during film formation to enhance antibacterial properties, drug carrier capabilities, or environmental responsiveness, providing a reliable, high-performance film foundation for tissue engineering, smart films, and drug delivery systems.













