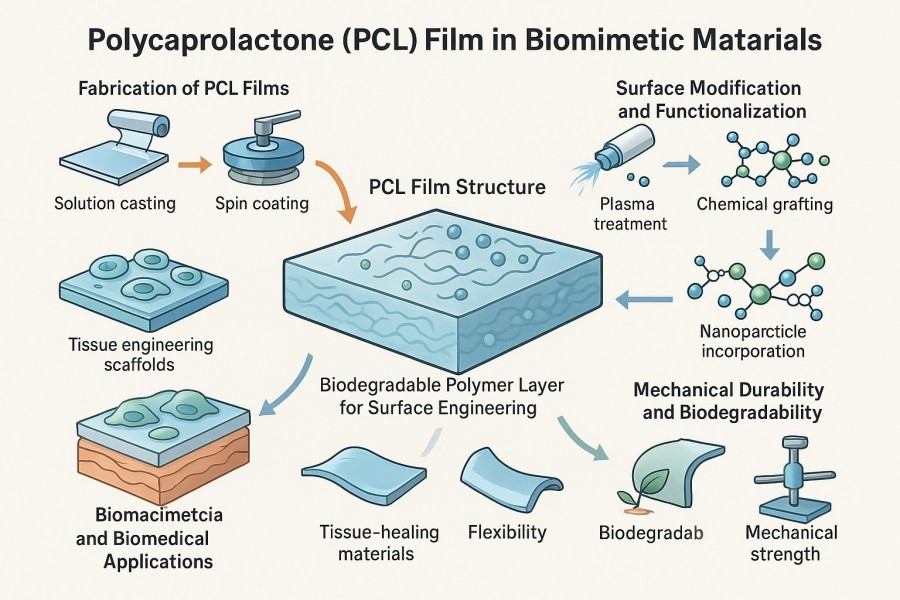
PCL film, made from polycaprolactone, is a biodegradable and versatile material. It offers excellent flexibility, ease of processing, and controllable degradation rates, making it ideal for applications in packaging, biomedical, and drug delivery systems.

- Home
-
Products
-
Monomers
- Acrylic Monomers
- Alcohol Monomers
- Allyl Monomers
- Amide & Imide Monomers
- Amine Monomers
- Anhydride Monomers
- Biodegradable Polymer Monomers
- Carboxylic Acid Monomers
- Cycloolefin
- Dendrimer Building Blocks
- Epoxide Monomers
- Halide Monomers
- Isocyanate Monomers
- Mercaptan Monomers
- Other Monomers
- Silicone Monomers
- Styrenic Monomers
- Vinyl Monomers
- Polymer Standards
-
Polymers
- Acid Functional Polymers & Salts
- Acrylic Polymers
- Alkene & Vinyl Polymers
- Amide and Imide Polymers
- Amine Functional Polymers & Salts
- Biodegradable Polymers
- Copolymers
- Dendrimers
- Epoxy Polymers
- Halogenated Polymers
- Natural Polymers & Derivatives
- Other Polymers
- Poly(ethylene glycol)s (PEGs) & Derivatives
- Polyester
- Polyether
- Silicones
- Styrenic Polymers & Derivatives
- π-Conjugated Polymers
- Reagents for Polymerization
-
Monomers
-
Custom Services
- Polymer Characterization Services
- Polymer Isolation and Purification
-
Custom Synthesis
- Copolymer Synthesis Services
- Polymer Additive Synthesis Services
- Polymer Initiator Synthesis Services
- Polymer Nanoparticle Synthesis
- Polymer Microsphere Synthesis
- Monomer Synthesis Services
-
Polymer Synthesis Services
- Atom Transfer Radical Polymerization Technology
- Reversible Addition-fragmentation Chain Transfer Polymerization Technology
- Nitroxide-mediated Polymerization Technology
- Ring-opening Polymerization Technology
- Ring-opening Metathesis Polymerization Technology
- Free Radical Polymerization Technology
- Living Cationic Polymerization (LCP) Technology
- Living Anionic Polymerization Technology
- Polymer Hydrogel Synthesis
- Polymer Micelle Synthesis
-
Polymer Modification Services
- Side/End Group Functionalization
- Side/End Group Protection
- Polymer Bioconjugation Services
- Biomimetic Materials
- Support
- About Us
- Contact Us