PLGA Nanoparticle Preparation
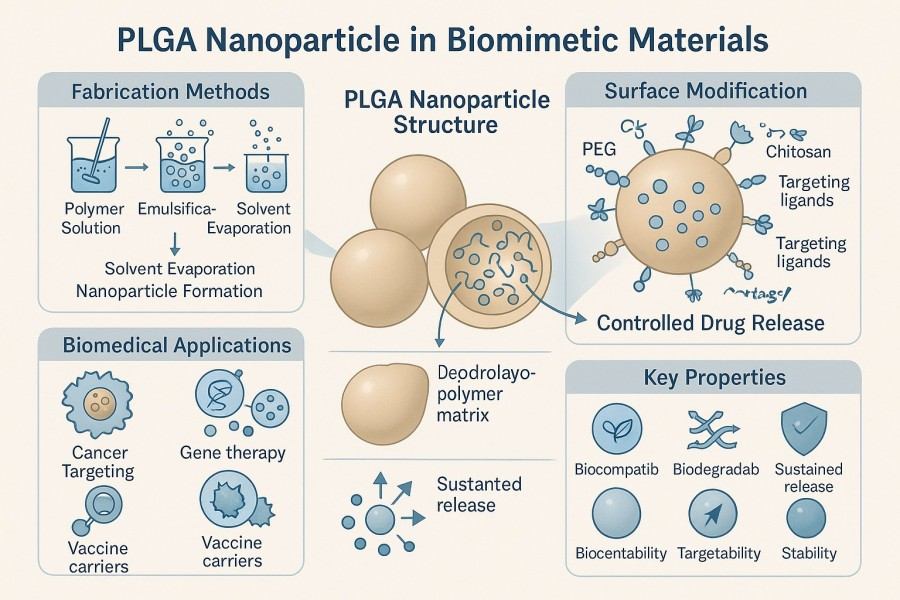
Poly(lactic-co-glycolic acid) (PLGA) nanoparticles are a class of biodegradable and biocompatible polymeric carrier materials, widely used in drug delivery, tissue engineering, diagnostic imaging, and biomimetic materials. Their degradation products, lactic acid and glycolic acid, can be metabolized into carbon dioxide and water in the body, offering excellent safety and environmental friendliness. With the advancement of nanomedicine and smart materials, PLGA nanoparticle preparation technology has become a key bridge connecting life sciences and materials science. By precisely controlling particle size, surface structure, molecular weight, and the lactic acid/glycolic acid ratio, materials with different degradation rates, drug release profiles, and biodistribution characteristics can be obtained. BOC Sciences provides customized synthesis, characterization, functional modification, and biomimetic application development services for PLGA nanoparticles, supporting both research and industrial innovation. We can tailor particle size, surface properties, and drug loading schemes according to scientific and industrial requirements, producing nanoparticles with high uniformity, stability, and controlled release. From laboratory research and small-scale trials to pilot-scale production, we provide full-process technical support and customized solutions to accelerate innovation projects.
What We Offer
BOC Sciences: High-Quality PLGA Nanoparticle Solutions
BOC Sciences possesses systematic design and preparation capabilities for PLGA nanoparticles, enabling the customization of various PLGA systems according to different structural features, drug loading methods, and application scenarios. Our offerings include drug carriers, functional composites, and biomimetic nanostructures, meeting diverse needs in research and industrial applications.
PEG–PLGA Nanoparticles
The PEG–PLGA system is a mainstream platform for drug delivery and long-circulating nanocarrier development.
- Long circulation and enhanced stability: PEGylation significantly reduces serum protein adsorption and immune recognition, extending in vivo circulation time.
- Controlled degradation: Adjusting the PEG-to-PLGA ratio achieves desired drug release profiles.
- Multifunctional modification support: Targeting ligands (antibodies, peptides) or fluorescent probes can be incorporated for precise delivery or imaging.
- Application-driven development: Suitable for anticancer drugs, vaccine carriers, and nucleic acid delivery systems.
PLGA–PVA Nanoparticles
Polyvinyl alcohol (PVA) is commonly used to stabilize PLGA nanoparticles. BOC Sciences offers multi-level dispersed, size-controllable PVA-coated PLGA nanoparticles.
- Stable emulsion system: Optimized PVA emulsification ensures uniform particle size and stable morphology.
- High drug encapsulation efficiency: Suitable for dual loading of small hydrophobic molecules and proteins.
- Excellent biocompatibility: PVA coating improves interface properties, reducing cytotoxicity.
- Scalable process: Supports emulsion–evaporation, microfluidics, and spray-drying for large-scale production.
PLGA–Chitosan Nanoparticles
Chitosan, a cationic natural polysaccharide, provides excellent surface charge control and mucosal adhesion properties to PLGA nanoparticles.
- Positively charged surface: Enhances interactions with cell membranes and mucosal tissues, promoting uptake.
- Improved bioadhesion and permeability: Suitable for oral, nasal, and pulmonary delivery systems.
- Controllable layer-by-layer assembly: Supports PLGA core–shell or multilayer coating designs.
- Green biodegradable system: All components are biocompatible and biodegradable, suitable for biomedical applications.
PLA–PLGA Nanoparticles
BOC Sciences provides multi-block copolymer nanoparticles based on polylactic acid (PLA) and PLGA.
- Adjustable copolymer ratio: Customizes degradation rates and mechanical properties according to PLA/PLGA ratios.
- Stable polymer chain structure: Suitable for long-acting and structurally reinforced nanocarriers.
- Covalent crosslinking or surface activation: Enables functional integration with biomolecules or metal nanostructures.
- Compatible with diverse drugs: Supports small molecules, proteins, peptides, and oligonucleotide encapsulation.
PLGA–Alginate Nanoparticles
PLGA–Alginate composite nanoparticles combine the advantages of synthetic polyesters and natural polysaccharides.
- Multilayer composite design: Alginate gelation enables layered or core–shell structures.
- Enhanced hydration and biocompatibility: Improves particle dispersibility and cell compatibility.
- Drug protection and controlled release: Alginate layer prevents premature drug leakage.
- Broad application: Suitable for tissue repair scaffolds, protein drug protection, and mucosal delivery.
Drug-Loaded PLGA Nanoparticles
For controlled release and targeted delivery of small molecules, macromolecules, and nucleic acids.
- Multiple loading modes: Encapsulation of hydrophilic or hydrophobic molecules, including chemotherapeutics, proteins, and siRNA.
- High encapsulation and release control: Optimized via emulsification or nanoprecipitation for efficiency and kinetics.
- Targeting functionality: Conjugation with antibodies, peptides, or glycan ligands for specific delivery.
- Sustained and long-acting systems: Suitable for continuous release or local administration strategies.
Surface-Modified PLGA Nanoparticles
Focuses on enhancing biocompatibility, stability, and targeting through surface chemistry or physical modification.
- PEGylation and hydrophobic layer control: Improves circulation stability and reduces immune recognition.
- Charge modulation and layer-by-layer assembly: Enhances cellular uptake or transmembrane transport.
- Multi-ligand co-modification: Enables multi-target recognition and synergistic effects.
- Controllable reactive interface: Supports bioconjugation, fluorescent labeling, and molecular tracking.
Functional Imaging and Magnetic PLGA Nanoparticles
For imaging, targeted diagnostics, and smart responsive systems.
- Fluorescent labeling and optical tracking: Incorporates BODIPY, FITC, or near-infrared dyes.
- Magnetic composite design: Fe₃O₄ or MnO₂ nanoparticles for magnetic responsiveness.
- Multimodal imaging: Enables MRI/fluorescence/CT analysis.
- Theranostic integration: Combines drug loading for diagnostic and therapeutic synergy.
Looking for Biomimetic Material Solutions?
From natural polymers to bio-inspired composites, BOC Sciences provides customized materials to accelerate your research and industrial applications.
Services
PLGA Nanoparticle Synthesis and Development Capabilities
At BOC Sciences, we specialize in the systematic development and customized preparation of PLGA nanoparticles. Leveraging our strong polymer chemistry foundation and comprehensive analytical systems, we provide full-process support—from molecular design to material scale-up—for drug delivery, biosensing, biomimetic materials, and tissue engineering. Our services focus not only on particle size control, surface modification, and functionalization but also cover diverse preparation and characterization techniques to ensure high uniformity, reproducibility, and biocompatibility.
1Polymer Synthesis and Custom Modification
- Provides PLGA polymers with adjustable lactic/glycolic acid ratios (e.g., 50:50, 75:25, 85:15).
- Offers different molecular weights and terminal groups (carboxyl/ester).
- Grafting or copolymerization of functional groups (PEG, carboxyl, amino, thiol) for surface modification.
2Nanoparticle Preparation and Structural Control
- Prepares PLGA nanoparticles via single/double emulsion, nanoprecipitation, microfluidics, etc.
- Precise control of particle size (20–500 nm), distribution (PDI < 0.2), surface charge, and morphology.
- Structural and purity verification via DLS, TEM, FTIR, NMR, GPC, HPLC.
3Functionalization and Targeting Design
- Surface modification strategies including PEGylation, ligand conjugation, antibody coupling, and lipid coating.
- Supports loading and controlled release of bioactive molecules (drugs, peptides, nucleic acids, proteins).
- Modifies interface properties via chemical bonding or adsorption to optimize cell interaction and biodistribution.
- Designs suitable for drug carriers, vaccine delivery, biomimetic scaffolds, and theranostic materials.
4Core Technologies for Nanoparticle Preparation
- Emulsion–Solvent Evaporation: Precise control of particle size and encapsulation via single or double emulsions.
- Nanoprecipitation: Narrow size distribution, simple operation, ideal for thermosensitive biomolecules.
- Spray Drying and Lyophilization: Enhances storage stability and dispersibility for long-term preservation and transport.
- Microfluidics: High-throughput, reproducible nanoparticle preparation, suitable for precise drug delivery system development.
Characterization
Synthesis and Characterization of PLGA Nanoparticles
BOC Sciences has established a multidimensional analysis and quality control system from raw materials to final products, covering physicochemical properties, structural analysis, stability, and functional performance verification.
| Analysis Item | Technique | Key Parameters |
|---|
| Particle Size & Distribution | Dynamic Light Scattering (DLS), Laser Particle Size Analyzer | Average particle size, PDI (polydispersity index), size uniformity |
| Morphology Characterization | Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) | Particle shape, surface smoothness, core–shell structure visualization |
| Chemical Structure Analysis | FTIR, NMR, GPC | Molecular structure verification, molecular weight and distribution, polymer end-group confirmation |
| Thermal Properties & Stability | Thermogravimetric Analysis (TGA), Differential Scanning Calorimetry (DSC) | Degradation temperature, glass transition temperature, thermal stability |
| Encapsulation Efficiency & Drug Loading | High-Performance Liquid Chromatography (HPLC), UV–Vis Spectroscopy | Drug content, encapsulation efficiency, drug loading capacity |
| Drug Release Performance | Dialysis method, sustained release curve analysis | Cumulative release rate, release kinetics constant |
| Surface Charge | ζ-Potential Analysis | Particle surface charge, colloidal stability, interfacial interaction |
Advantages
Technical Advantages in PLGA Nanoparticle Synthesis and Optimization

- Professional Technical Team: Our team possesses extensive experience in polymer and nanomaterial research, providing end-to-end technical support from polymer design to functional nanoparticle development.
- Multi-Platform Preparation Capabilities: We cover a wide range of preparation techniques, including emulsification, nanoprecipitation, microfluidics, and spray drying, to meet different particle size and application requirements.
- Strict Quality Control: Equipped with advanced instruments such as DLS, TEM, SEM, HPLC, and FTIR, we monitor the entire production process and ensure batch-to-batch consistency.
- Functionalization and Surface Modification: We support PEGylation, ligand grafting, polysaccharide composites, and copolymer modifications to enhance nanoparticle performance and biocompatibility.
- Customized Solutions: Tailored nanoparticle structures, encapsulation strategies, and drug release profiles are designed to meet specific project requirements.
- Biomimetic Material Development Support: We offer material–biointerface optimization, composite system design, and functional modification services to enhance stability in physiological environments.
- Pilot and Scale-Up Support: From laboratory-scale trials to kilogram-level production, we ensure smooth translation of research outcomes to industrial applications.
- Comprehensive Customer Support: Technical consultation, project progress updates, data analysis reports, and multilingual communication are provided to ensure smooth and efficient collaboration.
Service Process
Streamlined PLGA Preparation Workflow to Accelerate Your R&D
Our workflow covers every stage from needs assessment and material design to final nanoparticle optimization, ensuring clients receive reliable, reproducible products while maximizing the efficiency of research or industrial projects. With standardized procedures and professional team support, we respond quickly to client requirements and provide full technical guidance and customized solutions.
1Project Needs Assessment
- At the initial stage, we conduct detailed discussions with clients to understand project objectives, application scenarios, active substance properties, and specific performance requirements.
- We analyze drug physicochemical characteristics (hydrophilicity/hydrophobicity, stability, molecular weight, etc.) and define design parameters such as particle size, release schedule, and targeting needs.
- Feasibility assessments, expected outcome predictions, and project timelines with milestones are provided to ensure controlled development progress.
2Material Design and Custom Synthesis
- Based on assessment results, we provide a variety of PLGA polymer specifications and modification strategies to ensure nanoparticles meet application requirements.
- Appropriate lactide/glycolide ratios and molecular weights are selected to control degradation rates, with terminal group modifications or functionalization (carboxyl, amino, PEG, etc.) according to application needs.
- Customized block copolymer or polysaccharide composite designs are available to optimize drug loading efficiency and nanoparticle stability.

3Nanoparticle Preparation
- We utilize advanced preparation methods tailored to project characteristics to produce highly uniform and stable PLGA nanoparticles.
- Single or double emulsification methods are used for hydrophobic or hydrophilic drugs, while nanoprecipitation achieves narrow size distributions for thermosensitive drugs.
- Microfluidic technology ensures high-throughput and reproducible nanoparticle production; spray drying or freeze-drying facilitates storage and the preparation of dry powder or inhalable formulations.
4Performance Characterization and Analysis
- BOC Sciences has a comprehensive analytical platform to ensure each batch of nanoparticles meets design requirements in terms of size, morphology, chemical structure, and functionality.
- Particle size, PDI, and surface charge measurements confirm uniformity and colloidal stability; TEM/SEM provides morphological observation and core–shell structure visualization.
- FTIR, NMR, GPC, and other analyses verify chemical structure and molecular weight. Drug encapsulation efficiency, loading capacity, and release profiles are evaluated to ensure functional performance.
5Optimization and Custom Improvements
- Based on performance analysis, we provide process optimization and functional enhancement plans to achieve optimal application performance.
- Adjusting lactide/glycolide ratios or molecular weight optimizes degradation rates, and emulsification or precipitation parameters are refined to improve encapsulation efficiency and particle uniformity.
- Surface modification or targeted ligand grafting enhances biocompatibility and targeting, with multiple experimental validations to ensure batch-to-batch consistency.

6Delivery and Technical Support
- At the completion stage, we deliver the full nanoparticle product and analysis report, providing ongoing technical support.
- Laboratory- and pilot-scale nanoparticle products are supplied to meet research or application development needs, accompanied by detailed analysis reports including size, morphology, encapsulation efficiency, and release profiles.
- Guidance for process scale-up, usage instructions, and follow-up technical consultation are provided to support project translation and commercialization.
Applications
Applications of PLGA Nanoparticles in Biomimetic Materials
Thanks to their excellent biodegradability, biocompatibility, and functionalization potential, PLGA nanoparticles have become a core platform for biomimetic material design and biomedical applications. By precisely controlling particle size, surface properties, and polymer composition, PLGA nanoparticles enable controlled drug release, cellular delivery, and functional scaffold construction, offering reliable support for innovative biomimetic materials. Typical applications in key areas include:
Drug Delivery and Controlled Release Systems
PLGA nanoparticles provide controlled in vivo degradation and sustained drug release, widely used for anticancer drugs, anti-infectives, and small-molecule therapeutics.
- Degradation rates can be precisely tuned by adjusting lactide/glycolide ratios and molecular weight, achieving release periods from days to months.
- Particle size and surface properties optimize drug absorption and distribution, enhancing bioavailability.
- Both hydrophilic and hydrophobic drugs, as well as biomacromolecules, can be encapsulated and co-delivered.
- Targeted ligands or PEG modifications enable precise localization and controlled release in tumors or specific tissues.
Tissue Engineering and Regenerative Medicine
PLGA nanoparticles are combined with composite scaffolds and biomimetic extracellular matrices for bone, cartilage, and skin regeneration.
- Blending with natural polymers such as collagen, gelatin, or alginate creates a microenvironment closer to native tissue.
- Nanoparticles provide localized drug release to direct growth factors or signaling molecules.
- Adjusting particle size, morphology, and surface modifications supports cell adhesion, proliferation, and differentiation.
- Suitable for constructing 3D scaffolds or microenvironment models, supporting in vitro tissue studies or in vivo implantation.
Vaccine and Immune Delivery
As carriers for antigens or nucleic acids, PLGA nanoparticles enhance immune responses and are a focus of novel vaccine development.
- Capable of encapsulating protein antigens, mRNA, or DNA for sustained release and immune stimulation.
- Nanoparticles protect labile biomolecules from early degradation in vivo.
- Particle size, surface charge, and ligand modification optimize dendritic cell uptake and antigen presentation.
- Can be combined with adjuvants for targeted, long-lasting vaccine delivery strategies.
Medical Imaging and Diagnostics
PLGA nanoparticles serve as multifunctional imaging carriers for tumor detection, angiography, and multimodal imaging.
- Can load fluorescent dyes, MRI contrast agents, or radioisotopes for multimodal imaging.
- Particle size and surface modifications optimize in vivo distribution, circulation time, and targeting.
- Supports the development of nanoparticle–scaffold composites for tissue localization and functional analysis.
- Enables early disease detection and accurate diagnosis, facilitating both research and clinical applications.
Antibacterial and Wound Healing Materials
PLGA nanoparticles can serve as antibacterial drug carriers for chronic wounds, burns, or surgical implant surface modification.
- Enables local sustained drug release, extending antibacterial action and reducing repeat dosing.
- Nanoparticles can be combined with scaffolds or membrane materials to create biomimetic repair environments.
- Particle size and surface charge adjustments optimize drug penetration and tissue absorption.
- Supports personalized wound treatment strategies, integrating targeted release and functional surface modification.
Gene and Nucleic Acid Delivery
PLGA nanoparticles can protect and deliver nucleic acid molecules, enabling gene therapy and gene regulation applications.
- PLGA nanoparticles can deliver mRNA, siRNA, DNA, and other nucleic acids for gene therapy or editing.
- They protect nucleic acids from in vivo degradation, improving transfection efficiency.
- Surface modifications with targeting ligands or cationic polymers enhance cellular uptake and tissue specificity.
- Applicable in tumor, genetic disease, or immunomodulation research and applications.
FAQs
Frequently Asked Questions
-
What is PLGA nanoparticles?
PLGA nanoparticles are nanoscale particles composed of poly(lactic-co-glycolic acid), a biodegradable and biocompatible copolymer. They are widely used as drug delivery carriers, tissue engineering scaffolds, and vaccine or gene delivery systems. PLGA nanoparticles can encapsulate small molecules, proteins, peptides, or nucleic acids, providing controlled release, protection of active agents, and targeted delivery capabilities. Their degradation rate and surface properties can be tailored by adjusting the lactide/glycolide ratio, molecular weight, and surface modification.
-
What are the advantages of PLGA nanoparticles?
Biodegradability and biocompatibility: PLGA degrades into lactic acid and glycolic acid, naturally metabolized in the body.
Controlled drug release: Release kinetics can be tuned from days to months.
Versatility: Encapsulation of hydrophilic and hydrophobic drugs, proteins, peptides, and nucleic acids.
Targeted delivery potential: Surface modification enables tissue- or cell-specific targeting.
Stability enhancement: Protects sensitive drugs from enzymatic degradation and premature clearance.
Scalable production: Suitable for lab, pilot, and industrial-scale manufacturing.
-
How to make PLGA nanoparticles?
Several methods are commonly used depending on the drug type and desired particle properties:
Emulsion–solvent evaporation: Dissolve PLGA and drug in an organic solvent, emulsify in water with surfactant, then evaporate solvent to form nanoparticles.
Nanoprecipitation: PLGA is dissolved in a water-miscible solvent and added to the aqueous phase, causing rapid nanoparticle precipitation.
Double emulsion (W/O/W): Suitable for hydrophilic drugs; an aqueous drug solution is emulsified in PLGA organic solution, then re-emulsified in water.
Spray drying or microfluidics: For precise particle size control, high-throughput production, or dry powder formulations.
Surface modification: PEGylation, ligand attachment, or coating with chitosan/alginate can enhance targeting, stability, or cellular uptake.
-
What types of drugs can be loaded into PLGA nanoparticles?
PLGA nanoparticles are highly versatile and can carry small molecules, peptides, biomacromolecules, proteins, and nucleic acids. By adjusting particle size, surface charge, and polymer composition, they enable stable encapsulation, protection from degradation, targeted delivery, and controlled release for drug development and biomimetic material applications.
-
How to control the degradation rate of PLGA nanoparticles?
Degradation rates can be precisely controlled by adjusting lactide/glycolide ratios, molecular weight, terminal groups, and particle size. Higher lactide content slows degradation, while higher glycolide content accelerates it. Particle size and surface modifications also influence hydrolysis, allowing drug release from days to months, adaptable to different therapies and biomimetic applications.
-
Are PLGA nanoparticles biocompatible?
Yes, PLGA is FDA-approved for absorbable sutures and implantable materials, with excellent biocompatibility. Its degradation products, lactic acid and glycolic acid, are naturally metabolized, causing no significant toxicity or immune response, making it suitable for drug delivery, tissue engineering, vaccine delivery, and biomimetic materials.
-
Does BOC Sciences support scale-up production?
We offer scalable production services from lab to pilot scale, supporting research validation and commercial development. Clients receive uniform, stable PLGA nanoparticles along with complete quality control and analytical reports. Our team can optimize production processes to support batch-scale manufacturing.
-
What biomimetic applications are PLGA nanoparticles used for?
PLGA nanoparticles are widely used in biomimetic materials, including extracellular matrix mimics, tissue repair scaffolds, smart drug carriers, antibacterial coatings, and multimodal imaging systems. Their controlled degradation and functional surfaces allow precise delivery of drugs or signaling molecules, simulating native tissue microenvironments and supporting tissue engineering, regenerative medicine, and drug delivery research.