Polycaprolactone Microsphere Preparation
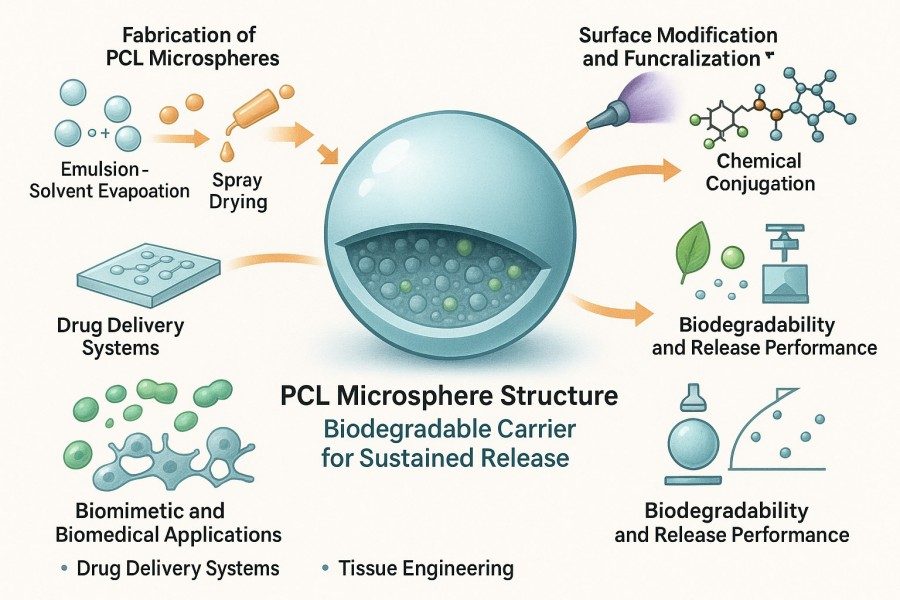
Polycaprolactone (PCL) microspheres are spherical or near-spherical particles composed of the biodegradable synthetic polymer polycaprolactone. They are commonly used to encapsulate, carry, or protect active substances such as drugs, growth factors, or cells, with particle sizes typically ranging from 1 μm to several hundred micrometers. With PCL's excellent biocompatibility, biodegradability, low toxicity, superior mechanical properties, and controllable degradation rate, PCL microspheres have become a highly promising and widely studied material in biomedical fields including drug delivery systems, tissue engineering, cosmetic fillers, and embolization therapy. BOC Sciences offers end-to-end custom PCL microsphere services tailored to your project needs—from raw material selection, polymerization control, particle size regulation, and surface modification to application performance evaluation. We provide comprehensive analytical testing and technical support to help clients optimize microsphere formulations and application strategies, enabling efficient translation from R&D to practical use. Whether for controlled drug release, tissue engineering scaffolds, or functional biomimetic materials, BOC Sciences delivers tailored microsphere solutions to meet a wide range of scientific and industrial requirements.
What We Offer
Partner with BOC Sciences for End-to-End PCL Microsphere Development
Our platform supports multiple structural systems, including solid, hollow, core-shell, porous, and composite microspheres. By precisely controlling molecular weight, porosity, particle size distribution, and surface chemistry, we provide full-process support from basic research to functional carrier development. We are capable of producing both nano- and microscale PCL microspheres, with flexible design of structural morphology and release behavior according to the characteristics of drugs, cells, or growth factors.
Pure PCL Microspheres
- Prepared using techniques such as single emulsion, spray drying, and microfluidics, enabling precise control over particle size and distribution.
- Mechanical properties and degradation cycles can be optimized based on molecular weight, crystallinity, or solvent systems.
- Structural characterization (FTIR, DSC, SEM, etc.) and release kinetics assessment are provided.
- Suitable for drug delivery, bone repair fillers, and long-term scaffold applications.
PCL Copolymer Microspheres
- Copolymer systems with different molar ratios can be designed to achieve tunable degradation rates and drug release profiles.
- Offers development of various copolymer formulations including PCL-PLGA, PCL-PEG, and PCL-PLA.
- Enables dual-drug loading or hydrophilic–hydrophobic co-encapsulation strategies.
- Supports small-scale scaling and process optimization for pharmaceutical and tissue engineering research.
PCL Composite Microspheres
- Can incorporate materials such as hydroxyapatite (HA), silica, silver nanoparticles, or Fe₃O₄ to achieve reinforcement, antimicrobial, or magnetic responsiveness.
- Offers organic–inorganic or polymer composite systems such as PCL/PLGA, PCL/Chitosan, and PCL/Gelatin.
- Customizable structures (solid, hollow, porous, or core-shell) to meet various drug carrier or cell scaffold requirements.
- Provides structural and performance evaluation, including porosity, release behavior, and biocompatibility testing.
Surface-Functionalized PCL Microspheres
- Introduces active functional groups such as carboxyl, hydroxyl, or amino groups to enhance hydrophilicity and cell adhesion.
- Offers biomolecule grafting and conjugation services (peptides, antibodies, glycans, PEG, etc.).
- Enables design of targeting, stimuli-responsive, or antimicrobial properties.
- Utilizes plasma treatment, chemical modification, or self-assembly to ensure modification stability and reproducibility.
Nano PCL Microspheres
- Particle size controlled in the range of 50–500 nm with uniform and adjustable distribution.
- Supports nano drug carriers, gene delivery, and intracellular delivery system designs.
- Can be combined with PLGA or liposome materials to enhance drug loading and release control.
- Provides Zeta potential, particle size distribution, encapsulation efficiency, and in vitro release testing services.
Micro PCL Microspheres
- Supports custom porous, hollow, and core-shell structures for drug sustained release or tissue filling.
- Can load various growth factors or anti-inflammatory drugs for bone, cartilage, and skin repair.
- Enables scalable production and batch consistency verification, supporting R&D to pilot-scale needs.
- Provides evaluations of formability, degradation behavior, and in vivo compatibility.
Looking for Biomimetic Material Solutions?
From natural polymers to bio-inspired composites, BOC Sciences provides customized materials to accelerate your research and industrial applications.
Services
PCL Microsphere Development and Manufacturing Capabilities
BOC Sciences delivers one-stop services covering material development, structural optimization, performance validation, and scalable manufacturing. Whether for scientific exploration, preclinical validation, or industrial scale-up, we provide highly stable and reproducible PCL microsphere solutions to support efficient innovation in biomimetic materials and biomedical products.
- Custom synthesis of PCL polymers with specific molecular weights and distributions to achieve targeted degradation rates and mechanical properties.
- Provides purification of PCL monomer (ε-caprolactone), ring-opening polymerization, and copolymer system design and synthesis.
- Capable of molecular structure control for copolymer systems such as PCL-PLGA, PCL-PEG, and PCL-PLA.
- Offers polymer property analysis (GPC, NMR, DSC, FTIR) to ensure controllable material characteristics.
2Custom Preparation of PCL Microspheres
- Provides multiple core processing routes: single/double emulsion evaporation, microfluidics, electrospray, spray drying, etc.
- Achieves precise particle size control from 50 nm to 1000 μm for nano carriers and micro implantation systems.
- Can produce various structural types: solid, hollow, core-shell, porous, composite, or hierarchical structures.
- Optimizes drug loading and release performance by adjusting polymer concentration, emulsification parameters, and solvent systems.
3Functionalization and Surface Modification
- Surface functionalization introducing carboxyl, amino, or hydroxyl groups to enhance hydrophilicity and cell compatibility.
- Conjugation of targeting molecules, bioactive substances, or antibodies for precise delivery and recognition.
- Development of PCL composite microspheres, e.g., PCL/HA, PCL/Fe₃O₄, PCL/PLGA, achieving magnetic, conductive, or antimicrobial functions.
- Utilizes plasma treatment, self-assembly, or graft polymerization to ensure surface modification stability and uniformity.
4Process Optimization and Scale-Up Production
- Systematic optimization of emulsification rate, solvent system, drying process, and encapsulation efficiency.
- Provides scalable process solutions for continuous production from milligram to kilogram levels.
- Establishes comprehensive quality control and validation systems to ensure batch consistency and traceability.
- Offers pilot-scale equipment design and process transfer consultation to accelerate industrialization.
Characterization
PCL Microsphere Analysis and Quality Control
BOC Sciences has established a comprehensive analytical and quality control system covering full-process testing from raw material verification to finished product characterization. Our advanced characterization platform allows systematic evaluation of microsphere morphology, structure, particle size distribution, surface properties, composition, degradation behavior, and biological performance, helping clients ensure product consistency and stability.
| Category | Testing Item | Analytical Method | Purpose / Description |
|---|
| Physicochemical Characterization | Molecular weight (Mn, Mw, PDI) | Gel Permeation Chromatography (GPC) | Evaluate polymer chain length distribution and uniformity |
| Thermal properties (Tg, Tm, Td) | DSC/TGA | Assess melting point, glass transition, and thermal stability |
| Chemical structure | FTIR/NMR | Confirm polymer structure and monomer composition |
| Morphology & Size Distribution | Particle size and distribution | Dynamic Light Scattering (DLS)/Laser Diffraction | Determine microsphere uniformity and dispersion |
| Surface morphology | SEM/TEM | Observe microsphere surface roughness and pore structure |
| Density and porosity | Pycnometry/BET | Evaluate internal structure and specific surface area |
| Functional & Compositional Analysis | Drug or biomolecule loading content | UV-Vis/HPLC | Quantify encapsulated active ingredients |
| Release profile testing | In vitro release assay | Assess controlled-release performance |
| Hydrophilicity and contact angle | Contact Angle Measurement | Evaluate surface wettability and modification effects |
| Biocompatibility & Degradability | In vitro degradation test | Mass loss/GPC tracking | Determine degradation rate under physiological conditions |
| Cytotoxicity assay | MTT/Cell viability test | Evaluate biological safety and cell compatibility |
Advantages
Professional PCL Microsphere Solutions & Service Benefits

- Professional Technical Team: With extensive experience in polymer and nanomaterial R&D, we provide full-process technical support from polycaprolactone design and copolymer development to microsphere performance optimization.
- Advanced Preparation Platform: Equipped with multiple microsphere fabrication techniques, including emulsion, spray drying, and microfluidics, enabling precise control of particle size, structure, and morphology.
- Customized R&D Capabilities: We develop PCL microspheres with varying molecular weights, functional groups, or composite structures according to client needs, supporting applications in drug delivery, tissue engineering, and more.
- Strict Quality Control System: A comprehensive analytical and validation process is in place, ensuring traceability from raw materials to finished products and guaranteeing consistency and reliability across batches.
- Functionalization and Composite Support: Offers diverse surface modification, blending, and copolymerization strategies to develop bioactive, biodegradable, or controlled-release PCL microspheres.
- Process Scale-Up and Production Experience: Capable of translating laboratory-scale preparation to pilot and industrial-scale production, providing scalable manufacturing solutions for clients.
- Full-Cycle Customer Support: Continuous guidance and technical communication throughout project design, experimental validation, and product optimization to ensure efficient project progress and successful outcomes.
Service Process
Your End-to-End Guide to PCL Microsphere Custom Services
BOC Sciences has established a systematic, traceable PCL microsphere preparation and development workflow, ensuring that each step—from requirement analysis to product delivery—meets scientific standards and client objectives. Through modular management and precise control, we provide full-process support for efficient development, stable production, and predictable performance.
1Requirement Analysis and Project Design
At the project initiation stage, our technical team works closely with clients to clarify target applications (e.g., drug delivery, tissue repair, or biomimetic material development) and functional requirements. By analyzing the properties of the target molecules, carrier parameters, and process feasibility, we formulate customized R&D plans, including PCL type, particle size range, structural features, and expected degradation profile. All plans are data-driven and application-oriented, ensuring scientifically sound and implementable designs.
2Material Selection and Polymerization Optimization
BOC Sciences possesses extensive expertise in polymer synthesis and copolymerization, selecting PCL materials with appropriate molecular weights, terminal groups, or comonomers according to project requirements. By controlling polymerization temperature, catalyst ratios, and reaction time, we achieve precise regulation of molecular weight distribution, branching, and crystallinity. Additionally, we can develop special-structured PCL copolymers or composite systems to enhance mechanical strength, biodegradation rates, or drug compatibility.

3Microsphere Preparation and Structural Control
During microsphere fabrication, we employ multiple core techniques, including emulsion solvent evaporation, spray drying, membrane emulsification, and microfluidic molding. By controlling emulsification conditions, solvent ratios, and stirring speed, we ensure uniform particle size and controllable distribution. We can produce solid, hollow, porous, or core-shell microspheres according to project requirements, offering diverse morphologies and precise structural PCL microsphere solutions.
4Functionalization and Composite Development
For specific applications, BOC Sciences provides multi-level functionalization and composite design support. Chemical modification, blending, or grafting can introduce specific functional groups to adjust surface hydrophilicity, biocompatibility, or targeting. We also incorporate drugs, proteins, peptides, and nanofillers (e.g., HA, SiO₂) into the PCL matrix to develop multifunctional composite microspheres for controlled release, tissue repair, or responsive material applications.
5Performance Testing and Quality Validation
Our comprehensive testing platform conducts characterization of particle size distribution, surface morphology (SEM/TEM), thermal properties (DSC/TGA), and chemical composition (FTIR/NMR). For drug-loaded or functionalized microspheres, encapsulation efficiency, release profiles, and biocompatibility tests are performed. Each sample is validated against strict quality standards to ensure batch stability and compliance with project requirements.

6Process Scale-Up and Product Delivery
After laboratory preparation and validation, we conduct process scale-up studies, optimizing emulsification conditions, solvent recovery, and drying processes to ensure reproducibility and industrial feasibility. BOC Sciences provides pilot and bulk production services, maintaining structural consistency and performance stability during scale-up. Final delivery includes product samples, test reports, and detailed technical documentation to support subsequent application development or regulatory filing.
Applications
Major Applications of PCL Microspheres in Biomimetic Materials
Due to their excellent biocompatibility, controllable degradation, and mechanical stability, PCL microspheres are widely applied in biomimetic material development. By precisely tuning particle size, structure, and surface functionalization, PCL microspheres can mimic natural tissue structures or achieve specific biological functions, meeting the needs of drug delivery, tissue repair, and biomimetic material design. Key application areas include:
Drug Delivery Systems
- Sustained and Controlled Release: PCL microspheres can encapsulate small molecule drugs, proteins, or peptides for continuous release, prolonging therapeutic effects.
- Targeted Delivery: Surface-functionalized microspheres enable tissue- or cell-specific targeting, improving drug efficacy and reducing side effects.
- Combination Delivery: Core-shell or porous microspheres can simultaneously carry multiple drugs for synergistic therapy.
Tissue Engineering Scaffolds
- Mimicking the Natural Cellular Microenvironment: PCL microspheres with porous or composite structures provide 3D support for cell adhesion, proliferation, and differentiation.
- Controlled Degradation Support: Degradation rates can be tuned to allow scaffolds to gradually replace natural tissue during regeneration.
- Multifunctional Composite Scaffolds: Combining with hydroxyapatite, collagen, or gelatin enhances mechanical performance and bioactivity.
Bioactive Biomaterials
- Promoting Cell Adhesion and Proliferation: Surface-modified or functionalized microspheres can incorporate growth factors, peptides, or glycans to enhance cell-material interactions.
- Antimicrobial and Immunomodulatory Functions: Loading silver nanoparticles or antimicrobial agents enables development of microspheres for wound healing or implantable materials.
- Stimuli-Responsive Materials: pH-, temperature-, or magnetically-responsive PCL microspheres can be used in smart biomimetic material design for environment-triggered functionality.
Soft-Hard Tissue Interface Materials
- Composite Multilayer Structures: PCL microspheres can form scaffolds at soft-hard tissue interfaces, achieving mechanical and structural gradients.
- Nano/Microscale Gradient Design: Combining microspheres of different sizes and functionalization mimics the hierarchical structure of natural tissue.
- Functional Zonal Delivery: Microspheres in different layers can carry distinct growth factors or drugs for spatially controlled release.
Implantable and Regenerative Materials
- Filling Bone Defects or Soft Tissue Cavities: Microscale PCL microspheres can form injectable or moldable fillers for localized tissue repair.
- Biodegradable Implants: Microspheres gradually degrade with tissue regeneration, eliminating the need for secondary surgery.
- Personalized Scaffold Design: Composite or 3D-printed microspheres enable biomimetic materials with specific shapes and functions.
Drug Screening and Cell Culture Platforms
- Mimicking 3D Cellular Environments: Microspheres can construct 3D culture scaffolds, enhancing physiological relevance for cell behavior studies.
- High-Throughput Drug Testing: Controlled drug release from microspheres allows reliable in vitro drug screening models.
- Tunable Microenvironments: Microsphere surface modification or porosity can regulate local chemical and mechanical conditions.
FAQs
Frequently Asked Questions
-
What are PCL microspheres?
PCL microspheres are biodegradable spherical particles made from polycaprolactone (PCL), widely used in drug delivery systems, tissue engineering, and biomimetic material research. With high biocompatibility and controllable degradation, they are essential for sustained-release drugs and tissue scaffolds.
-
How to prepare PCL microspheres?
PCL microspheres are typically prepared using solvent evaporation, double emulsion, spray drying, or microfluidic techniques. Different methods allow control over particle size, drug loading, and surface properties to meet diverse biomedical applications and controlled-release requirements.
-
How to control PCL microsphere size and distribution?
Particle size can be precisely regulated by adjusting polymer concentration, solvent type, stirring speed, and emulsification conditions, ranging from nanoscale to microscale. Optimizing particle size enhances drug release profiles and tissue engineering performance.
-
What are the benefits of PCL microspheres in drug delivery?
PCL microspheres enable long-term sustained and targeted drug release, protecting drug activity and reducing side effects. Copolymerization or surface modification allows precise tuning of release rates to improve therapeutic efficacy.
-
Which biomedical applications are PCL microspheres suitable for?
PCL microspheres are widely applied in drug delivery systems, tissue engineering scaffolds, cell carriers, and vaccine delivery. Their biodegradability and processability make them particularly effective in bone repair, soft tissue engineering, and targeted cancer therapy.
-
How to control the degradation rate of PCL microspheres?
Degradation rate can be adjusted by modifying polymer molecular weight, copolymer ratio, crystallinity, and surface properties. This allows PCL microspheres to support sustained drug delivery or tissue repair from weeks to months.













