Chitosan Microsphere Preparation
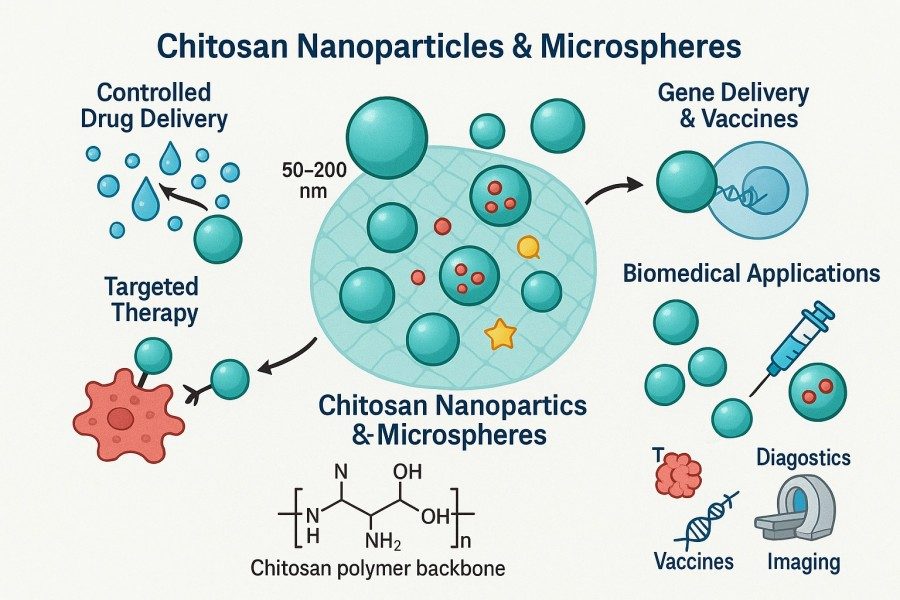
Chitosan microspheres, as a particulate form of chitosan derivatives, combine the biological functionality of chitosan with the high surface area, controlled release, and multifunctional loading characteristics of microsphere systems. This unique combination has attracted widespread attention and in-depth research in the field of biomimetic materials. They can not only mimic the structural environment of natural tissues but also provide customizable performance for different applications through chemical modification, crosslinking, and composite strategies. BOC Sciences specializes in the custom preparation and development of chitosan microspheres, offering high-quality, one-stop services for research institutions and industrial clients. Leveraging a mature polymer synthesis platform and diverse preparation techniques, we precisely control microsphere size, pore structure, crosslinking density, and surface functionalization, providing tailored solutions for various applications. Whether solid, hollow, porous, or hydrogel microspheres, we offer end-to-end technical support from formulation design, process development, and structural characterization to pilot-scale production, enabling clients to efficiently develop high-performance microsphere products for biomimetic materials, drug delivery, tissue engineering, and functional coatings.
What We Offer
Chitosan Microspheres Supported by BOC Sciences
We can flexibly design formulations and process routes according to different research directions, structural requirements, and application scenarios, achieving precise control over key parameters such as particle size distribution, pore structure, shell thickness, and surface functionality. Whether basic, structural, or highly functionalized chitosan microspheres, we provide full-process technical support from raw material selection, process development, structural characterization, to scale-up production. Our service portfolio includes solid, hollow, porous, composite, crosslinked, and hydrogel microspheres, meeting diverse needs across high-end applications in biomimetic materials, tissue engineering, drug delivery, and functional coatings.
Standard Chitosan Microspheres
- Prepared via solution gelation, emulsion crosslinking, or spray drying, forming uniform and structurally stable microspheres.
- Suitable for basic material research, carrier construction, and general controlled-release system development.
- Chitosan concentration and acidic solvent ratio can be adjusted to control the spheroidization process and particle size distribution.
Solid Chitosan Microspheres
- Produced through homogeneous crosslinking or spray drying, featuring dense internal structure and excellent mechanical strength.
- Can serve as scaffolds for tissue engineering or as the backbone for long-term controlled-release carriers.
- Supports various molecular weights of chitosan and crosslinking systems to ensure structural stability and biocompatibility.
Hollow Chitosan Microspheres
- Fabricated via templating or phase separation techniques, achieving hollow structures with controllable shell thickness, high surface area, and large internal volume.
- Suitable for efficient encapsulation and targeted release of drugs, enzymes, or bioactive molecules.
- Often used in lightweight biomimetic coatings and composite materials to enhance functional diversity.
Porous Chitosan Microspheres
- Formed through phase separation, bubble templating, or controlled crosslinking to create uniform internal networks.
- High surface area and permeability make them ideal for cell encapsulation, enzyme immobilization, and controlled-release systems.
- Can be combined with alginate to form chitosan alginate microspheres, further enhancing pore structure stability and biocompatibility.
Crosslinked Chitosan Microspheres
- Chemically crosslinked using glutaraldehyde or ionic crosslinkers to improve chemical resistance and structural stability.
- The resulting chitosan glutaraldehyde microspheres exhibit excellent mechanical strength and tolerance, suitable for long-term engineering applications.
- Crosslinking density and reaction time can be adjusted to meet specific release rates and physical property requirements.
Composite Chitosan Microspheres
- Chitosan is combined with natural or synthetic polymers such as alginate, gelatin, or PEG to form multilayer coatings or interpenetrating networks.
- Chitosan alginate microspheres are a typical example, leveraging alginate's gel properties and chitosan's cationic features.
- Suitable for tissue engineering, biomimetic coatings, and multi-phase controlled-release systems.
Chitosan Hydrogel Microspheres
- Formed via physical or chemical crosslinking into a three-dimensional hydrogel network, maintaining high water content and soft structure.
- Exhibits excellent biocompatibility, suitable for cell encapsulation, tissue engineering, and drug-controlled release.
- Supports smart hydrogel designs responsive to temperature, pH, or ions, meeting complex biomimetic material requirements.
Functionalized Chitosan Microspheres
- Surface-modified or grafted with active groups, fluorescent probes, ligands, or targeting molecules to achieve specific functions.
- Can be further combined with crosslinking and composite strategies to design microspheres with smart response, specific recognition, or traceable capabilities.
- Applicable to high-end biomedical materials, imaging probes, and intelligent biomimetic systems.
Looking for Biomimetic Material Solutions?
From natural polymers to bio-inspired composites, BOC Sciences provides customized materials to accelerate your research and industrial applications.
Services
High-Quality Chitosan Microsphere Development Services
BOC Sciences focuses on the custom preparation and development of chitosan microspheres, leveraging advanced polymer processing technologies and comprehensive process systems to provide high-quality, one-stop technical support for research and industrial clients in the biomimetic materials field. We offer mature preparation methods, including solution gelation, emulsion crosslinking, and spray drying, enabling precise control of particle size distribution, pore structure, and surface properties to meet the performance requirements of various applications. Additionally, our R&D team provides raw material selection, formulation optimization, functional modification, and comprehensive structural and performance characterization tailored to specific client needs.
1Diverse Preparation Technology Platform
- Ionic Gelation: Gentle process, suitable for encapsulating thermosensitive bioactive substances.
- Emulsion Crosslinking: Produces uniform, structurally stable microspheres.
- Spray Drying: Suitable for scale-up production and rapid drying.
- Microfluidics & Freeze-Drying: Enables precise particle size control and construction of highly functional structures.
- Customizable particle sizes (1–500 μm), porosity, crosslinking degree, and surface chemistry according to application requirements.
2Precise Chemical Modification and Functionalization
- Surface grafting of functional groups (e.g., carboxyl, thiol, PEG, targeting ligands).
- Crosslinking density and charge regulation.
- Introduction of smart-responsive groups (pH, temperature, enzyme-responsive).
- Multi-material composites (e.g., chitosan-gelatin, chitosan-ceramic).
- Enables differentiated solutions for diverse biomimetic material systems.
3Comprehensive Characterization and Quality Control
- Particle size distribution and morphology analysis (DLS, SEM, TEM).
- Crosslinking degree and chemical structure characterization (FTIR, NMR, elemental analysis).
- Pore structure measurement (BET, mercury porosimetry).
- Encapsulation efficiency and release profile analysis (UV, HPLC).
- Strict quality management ensures reproducibility and stability across batches.
4Scale-Up from Lab to Pilot
- Process scale-up and parameter optimization.
- Batch consistency and production controllability verification.
- Product drying, purification, and long-term storage.
- Provides reliable technical support for exploratory research, small-scale trials, or early industrial validation.
Advantages
Benefits of Chitosan Microsphere Fabrication Services

- Diverse Preparation Techniques: We offer multiple preparation technologies, including solution gelation, emulsion crosslinking, spray drying, microfluidics, and freeze-drying. These methods enable precise control of microsphere particle size, pore structure, and shell thickness, supporting the production of solid, hollow, porous, and hydrogel microspheres.
- Precise Functionalization Design: We provide crosslinking modification, composite material integration, and surface functionalization services, including the preparation of chitosan glutaraldehyde microspheres, chitosan alginate microspheres, and chitosan hydrogel microspheres. Through chemical modification or ligand grafting, microspheres can be endowed with smart responsiveness, targeted recognition, and traceable functionality, meeting the needs of advanced biomimetic material development.
- Full-Process Customization and Optimization: Our services cover the entire workflow from raw material selection, formulation design, process development, and structural and performance characterization to laboratory trials, pilot studies, and scale-up production. This ensures that each batch of microspheres meets client expectations for performance, stability, and biocompatibility.
- Strict Quality Control and Characterization: Equipped with particle size analysis, porosity measurement, chemical structure characterization, and encapsulation/release testing, we ensure the reproducibility and high-quality standards of our microspheres.
- Flexible and Responsive Customer Support: Our technical team quickly provides solution optimization and troubleshooting based on client needs, supporting efficient development of projects in biomimetic materials, drug delivery systems, and tissue engineering.
Service Process
Chitosan Microsphere Preparation Service Workflow
Our service workflow covers the full cycle from project consultation and solution design to scale-up production, ensuring accuracy and efficiency at every step while meeting client requirements for particle size, pore structure, functionalization, and biocompatibility. Through rigorous process management and technical optimization, we can quickly translate client design concepts into high-performance microsphere materials, providing reliable support for biomimetic materials, drug delivery, and tissue engineering applications.
1Project Requirement Assessment and Consultation
- Communicate with clients about application scenarios, performance requirements, and microsphere types (solid, hollow, porous, hydrogel).
- Provide technical feasibility analysis and solution recommendations, including particle size range, crosslinking method, and functionalization needs.
2Raw Material Selection and Formulation Design
- Select appropriate chitosan types, molecular weights, and purity grades to ensure biocompatibility and processability.
- Design composite or functionalized formulations according to client needs, such as chitosan alginate microspheres or chitosan glutaraldehyde microspheres.

3Microsphere Preparation and Process Optimization
- Employ solution gelation, emulsion crosslinking, spray drying, microfluidics, or freeze-drying to produce uniform and structurally stable microspheres.
- Adjust process parameters to control porosity, shell thickness, and carrier properties, achieving customized performance.
4Functional Modification and Composite Treatment
- Provide surface grafting, active group introduction, and smart-responsive modifications.
- Develop chitosan hydrogel microspheres or other hydrogel microspheres to achieve smart release or targeted functions.

5Performance Characterization and Quality Testing
- Analyze particle size and morphology (DLS, SEM, TEM), and determine pore structure (BET, porosity analysis).
- Assess crosslinking degree, chemical structure, encapsulation efficiency, and release profile to ensure stability and reproducibility.
6Lab Trials, Pilot Studies, and Scale-Up
- Offer laboratory trials, pilot verification, and scale-up support according to R&D stage requirements.
- Ensure process control and consistent product quality, providing a reliable foundation for subsequent industrial applications.
Applications
Applications of Chitosan-Based Microspheres in Biomimetic Materials
Chitosan microspheres are applied in biomimetic materials for tissue engineering scaffolds, bio-coatings, controlled release systems for drugs and growth factors, smart-responsive materials, cell encapsulation, and microenvironment simulation. Their unique structures and functionalities allow them to mimic natural tissue environments, regulate bioactive substance release, and construct multi-scale biomimetic architectures, making them essential units in various biomimetic material designs.
Tissue Engineering and Regenerative Medicine
In tissue engineering, chitosan microspheres are used as cell growth scaffolds, tissue fillers, or growth factor carriers:
- Scaffold Material Filling and Repair: Incorporating chitosan microspheres into porous biomimetic scaffolds enhances overall surface area and pore connectivity, facilitating cell adhesion and nutrient exchange.
- Growth Factor Controlled Release: Microspheres can encapsulate growth factors such as BMP-2 or VEGF, regulating the local microenvironment via sustained release to promote bone, vascular, or soft tissue regeneration.
- Cell Carrier: Porous microspheres serve as three-dimensional culture matrices, simulating the extracellular matrix for cell encapsulation and tissue construction.
Drug and Signaling Molecule Delivery Systems
Chitosan microspheres are widely used in biomimetic drug delivery due to controlled release and excellent biocompatibility:
- Sustained and Targeted Delivery: Adjusting crosslinking density and pore size allows sustained drug release and localized high-efficiency action.
- Multi-Drug Co-Loading Systems: Microspheres enable simultaneous loading and gradient release of multiple drugs, simulating natural tissue signaling mechanisms.
- Smart-Responsive Delivery: pH-, temperature-, or enzyme-responsive modifications allow microspheres to trigger drug release in specific physiological environments, mimicking adaptive behavior.
Biomimetic Coatings and Interface Modification
Chitosan microspheres can also be used for functional coatings or surface modification:
- Antimicrobial Biomimetic Coatings: Leveraging the cationic nature of chitosan, microspheres can be fixed on material surfaces to form durable antimicrobial interfaces, mimicking natural skin defense mechanisms.
- Cell Adhesion and Tissue Integration: Distribution of microspheres on implants or scaffolds enhances cell adhesion and tissue integration.
- Smart-Responsive Interfaces: Combined with pH- or temperature-sensitive modifications, microspheres impart dynamic interface adjustment, simulating tissue adaptability.
Microenvironment Simulation and Biomimetic Structure Construction
Chitosan microspheres serve as building blocks for multi-scale biomimetic structures:
- Biomimetic Extracellular Matrix: Microspheres of different sizes and distributions can mimic the 3D arrangement of fibers and matrices in natural tissues.
- Layered Composite Structures: Combining microspheres with other natural or synthetic materials creates layered biomimetic structures, such as bone-cartilage interface materials.
- Cell Encapsulation and Local Microenvironment Regulation: Embedding cells or signaling molecules within microspheres reproduces tissue microenvironments, supporting complex tissue construction.
FAQs
Frequently Asked Questions
-
What are chitosan microspheres?
Chitosan microspheres are micron-sized spherical particles made from natural polysaccharide chitosan via physical or chemical methods. They offer tunable particle size, pore structure, and surface functionality, widely used in tissue engineering, drug delivery, and biomimetic materials for encapsulation, sustained release, and targeted delivery of bioactive substances.
-
What are the advantages of chitosan microspheres?
Chitosan microspheres feature excellent biocompatibility, biodegradability, tunable mechanical properties, controllable pore structure, and easy functionalization. These characteristics make them valuable for drug delivery, cell carriers, tissue engineering scaffolds, and intelligent biomimetic materials, supporting diverse functional design requirements.
-
How is the stability of chitosan microspheres?
The stability of chitosan microspheres can be enhanced through crosslinking, composites, or surface modification. Crosslinkers such as glutaraldehyde or ionic agents strengthen the structure, while porous and composite microspheres improve physicochemical tolerance, maintaining particle size, porosity, and functionality during storage and use.
-
How is the synthesis of chitosan microspheres carried out?
Chitosan microspheres can be synthesized via solution gelation, emulsion crosslinking, spray drying, or microfluidics. By adjusting chitosan concentration, acidic solvent ratio, and crosslinking conditions, particle size, pore structure, and drug-loading performance can be precisely controlled for customized functionality.
-
What are chitosan alginate microspheres?
Chitosan-alginate microspheres are formed by combining chitosan with alginate, integrating the cationic properties of chitosan and the gelation ability of alginate. They offer high biocompatibility, controllable porosity, and sustained release, commonly used as drug carriers and tissue engineering scaffolds.
-
What are chitosan glutaraldehyde microspheres?
Chitosan-glutaraldehyde microspheres are formed via glutaraldehyde crosslinking, significantly enhancing mechanical strength and chemical resistance. Suitable for long-term storage and drug carrier applications, crosslinking density can be adjusted to achieve desired release rates and structural stability.
-
How are chitosan microspheres used in drug delivery?
Chitosan microspheres enable sustained, targeted, and multi-drug release in drug delivery. Their tunable pore structure and surface functionalization allow efficient drug release at specific sites, reduce side effects, and support stable delivery of proteins, peptides, and small molecules, widely applied in biomimetic drug carrier systems.













